Abstract
Anesthesia-induced postoperative cognitive dysfunction (POCD) has been confirmed in elderly patients, while studies have shown that Nampt protein is critical for learning and memory. To better understand the mechanism of anesthesia-induced POCD, we studied the behavioral and biochemical changes in aged rats that were exposed to sevoflurane (Sev) and nitrous oxide (N2O) for 4 hours. Rats were randomly divided into control group and anesthesia group. The anesthesia group rats were given 1.3% Sev and 50% N2O for 4 hours, and controls with 50% O2 for same time. Morris Water Maze test was used to test the rat’s ability to learn and remember 24 hours exposure. The result shown that Sev-N2O anesthesia induced a significant deficit in short-term spatial learning acquisition and memory retention, but it had no significant deficit in long-term. After 48 hours Sev-N2O anesthesia, the neuronal apoptosis and the expression of Bax, PARP-1 in hippocampus of rats increased significantly, and the expression of Nampt, RelB decreased significantly. However, Nampt activators could reduce the apoptosis of hippocampal primary cells in vitro after 4 hours exposed with Sev-N2O. Thus, we believed that down-regulation of Nampt/RelB signaling was closely related to neuronal apoptosis in the hippocampus contributed to the neurotoxicity and cognitive dysfunction induced by general anesthesia with sevoflurane-nitrous oxide.
Keywords: Anesthesia, postoperative cognitive dysfunction, Nampt
Introduction
Postoperative cognitive dysfunction (POCD) is a cognitive impairment, which is caused by surgery and anesthesia. It is a transient disorder of memory, attention, language comprehension and social integration, and advanced age is a risk factor for the disease. The previous study [2,3] have showed that the incidence of POCD was 40% one week after operation in the elderly patients after non-cardiac surgery, and still more than 10% 3 months after operation. The memory impairment and the decrease of learning ability were the main clinical manifestations.
Although the pathogenesis and molecular mechanisms of POCD are not clear, the current study suggests that, POCD is a neurological deficit caused by a combination of multiple factors includes surgery, anesthesia and so on [4-6]. And neurodegenerative disease [7], inflammatory [8] and central nervous system degradation [9] are the current mainstream of the POCD pathogenesis hypothesis. Inhalation anesthesia plays an important role in anesthesia nowadays [10], while studies have shown that the anesthetic drugs desflurane, isoflurane and sevoflurane commonly used in inhalation anesthesia can cause transient cognitive impairment in the elderly patients [11-15]. Because of the common use of isoflurane and sevoflurane in inhalation anesthesia, animal studies are mainly focused on these two drugs.
Sevoflurane is commonly used in inhalation anesthesia clinically. It plays its role in anesthesia and forgetting by inhibiting N-methyl-D-aspartic acid receptor, and clinically, N2O is used in combination to reduce sevoflurane’s dosage. The previous studies have shown that transient inhalation of sevoflurane can cause transient reversible tau-protein phosphorylation, and long-term repeated inhalation of sevoflurane can damage the mouse’s spatial memory 1 month after surgery [16]. At the same time, Chen G et al. [17] showed that the endoplasmic reticulum stress-mediated apoptosis plays an important role in the neurodegenerative and cognitive impairment induced by sevoflurane inhalation. However, these studies all suggest that the cognitive impairment caused by seizethaether inhalation is short-term; this cognitive impairment may be associated with the decrease of neuron apoptosis in hippocampus region caused by oxidative stress, inflammatory response, etc [14,15,17].
Nicotinamide phosphoribosyl transferase (Nampt) is the rate-limiting enzyme of nicotinamide adenine dinucleotide (NAD+) remediation synthesis pathway, which is involved in cellular energy metabolism, apoptosis and autophagy, and plays a role in immune, inflammation and metabolic related diseases. The Nampt-NAD-sirtuin hypothesis is a new hypothesis about aging in recent years [18]. In the process of senescence of mammals, the expression of Nampt gradually decreased, resulting in the decrease of NAD content, thus inhibiting the activity of acetylated sirtuins, affecting the senescence process of eukaryotic. NAD can effectively protect neurons and inhibit apoptosis, whereas sirtuins can affect apoptosis, senescence, inflammation and metabolism by regulating the acetylation of histones and other cytokines [19,20]. It confirmed that loss of Nampt expression is one of the major causes to increase the formation of Alzheimer’s disease amyloid beta (Aβ) and plaque, increasing the expression of Nampt can significantly delay the development of neurodegenerative lesions [21,22].
Based on these, the present study was performed to determine whether Sev-N2O anesthesia in aged rats could reduce the short-term learning and memory ability, and evaluated the protective effect of Nampt/RelB pathway by Morris Water Maze, western blot and so on.
Methods and materials
Animal for research
The SD rat (16 months old and 24 hours old) was used to make animal models in present research, and was offered and fed (22±2°C, 12 h light cycle) by animal experimental center. All animal protocols were approved by the Animal Care and Use Committee in China Meitan General Hospital, and were confronted with the guidelines of National Institution of Health.
Anesthesia procedure
The Anesthesia group rat were given 1.3% sevoflurane (Baxter Medical Co., Ltd, China) and 50% N2O (Shanghai Haizhou Special Gas Co., Ltd, China) continuous inhalation in a custom transparent glass box for 4 hours, and the control group mice with 50% O2 (Shanghai Haizhou Special Gas Co., Ltd, China) for 4 hours. Electric blanket (Shanghai Yuyan Scientific Instrument Co., Ltd, China) was used to maintain rectal body temperature in (37.0±0.5)°C, the Multi-function monitor (Datex-Ohmeda, USA) was used to continuous monitoring of ETCO2 and inhalation anesthesia MAC values, and the oxygen saturation meter (Datex-Ohmeda, USA) is used to detect SpO2 in Anesthesia group. However, the control group did not monitor SpO2 to avoid stress.
Morris water maze text
The Morris water maze experiment was done by the researchers who did not know the rats group. Fist was 3 days for of adaptive training (water temperature and the surrounding environment).
The next 6 days (day 1 to day 6)was for place navigation test, the rats were randomly placed four times from the four quadrants into the water, and the swimming trajectories of the rats were recorded and analyzed by the automatic camera system and software (Shanghai Jigong Software Technology Co., Ltd, China). The rats boarded the platform and stayed for more than 5 seconds to be seen as successfully finding the hidden platform, then the automatic camera system automatically stopped recording. The next training began after the rats stay on the platform for 15 seconds to observe the surroundings. If the rats could not find the platform in 60 seconds, the automatic camera system also automatically stopped recording, and we would guide the rat to the platform, and recorded the escape latency as 60 seconds.
The space exploration test was conducted on day 7 and 14, respectively. The rats were put into the pool which was without the platform in random place twice, and recorded its swimming track in 60 seconds.
Western blot
The RIPA lysate buffer (Beyotime, China) with 1 mM PSMF (Beyotime, China) was used to extract the total cell protein, and Total Protein Extraction Kit-tissue (Beyotime, China) was used to extract total protein of brain tissue. The concentration of total protein was detected by BCAProtein Assay Kit (Beyotime, China), then the sample was boiled in 100°C water for 10 min. 75 mg total protein was loaded onto each lane, separated by 15% SDS-PAGE, and transferred onto a polyvinylidene difluoride membrane (Amersham Biosciences, UK). The membrane was blocked with 5% skimmed milk for 2 h, and then probed with Nampt (1:1000, Abcam, UK), RelB (1:1000, Abcam, UK), Bax (1:1000, Santa Cruz, USA), Bcl-2 (1:1000, Santa Cruz, USA), and β-actin (1:1000, Santa Cruz, USA) at 4°C overnight. Then washed twice with PBS buffer. HRP-goat anti rabbit or mouse antibody (1:5000, Santa Cruz, USA) was performed for detecting.
Paraffin sections and Nissl staining
After 48 hours of Sev-N2O anesthesia, some rats were executed, and the hippocampus was removed. The hippocampus is dehydrated by the following procedure: 75% ethanol overnight, 85% ethanol for 3 hours, 95% ethanol I for 1.5 hours, 95% ethanol II for 1.5 hours, 100% ethanol I for 1 hour, 100% ethanol II for 1 hour, xylene ethanol mixture for 25 minutes, xylene for 25 minutes. And then the waxing operation was paraffin for 2 hours. At last, slices were made by thermostat slicer (SLEE, CUT4062, Germany). The slices were warmed by 0.01 M PBS for 0.5 h, and rinse three times by PBS (10 min for 1 time) before Nissl staining.
Nissl staining was prepared as follows. Firstly, methyl violet dye smeared for 10-20 minutes. After washing the slices with water, Nissl differentiation solution was used to differentiation slices for 4-8 seconds. And then we could observe the cells by microscopy (BX51, OLYMPUS, Japan). Only intact neurons with a clearly defined cell body and nucleus were counted.
Immunofluorescence
10% milk and 0.5% Triton-100 which were diluted with PBS were used to block slices of rats hippocampalfor 1 hour at room temperature, and then in the primary antibody overnight at 4°C: PE-NeuN rabbit mAb (1:200, Abcam, UK), PE-Nampt mouse mAb (1:100, Abcam, UK), or RelB mouse mAb (1:100, Abcam, UK). After 3 times wash with PBS (10 minutes for one time), the second antibody was incubated for 4 hours: anti-rabbit or anti-mouse IgG (1:1000, Abcam, UK). DAPI (sigma, USA) was used to nuclear counterstaining for 30 minutes at room temperature.
Doubled staining with Annexin V-FITC and NeuN
Double staining with Annexin V-FITC and NeuN was used to detect the neuronal apoptosis in rat hippocampal. First labeling was with AnnexinV-FIFC by AnnexinV-FIFC labeling kit (Thermo Fisher, USA), then blocked with blocking solution as described above, incubated with primary anti-NeuN antibody (1:100, Abcam, UK) at room temperature for 2 hours and secondary antibody for 1 h.
Hippocampal primary neuronal cell cultures
Rats which was born within 24 hours was used to isolate hippocampal neuronal cell. Briefly, the rats were sacrificed and disinfected with 75% alcohol. And the rat hippocampus tissue was obtained by the following procedure which was in sterile conditions: cut the scalp skin, skull, open the brain field of vision with curved tweezers, carefully open the brain temporal Leaf cortex, exposed crescent hippocampus back, and sandwiched hippocampus tissue. We placed the rat hippocampus tissue in the ice bath D.hanks solution (Hyclone, USA), removed the microvascular, and fully cut the tissue. The same volume of 0.125% trypsin (Gibico, USA) was added to digest the rat hippocampus tissue in 37°C for 15 minutes, and stop digestion by adding fetal bovine serum (Hyclone, USA). Then, blow, filter and centrifuge cells (1000 rpm, 10 minutes, Eppendorf, Germany), resuspend cells with DMEM/F12 medium (+10% FBS, Hyclone, USA), and cultured the hippocampal Primary Neuronal Cell in 37°C incubator (Thermo fisher, USA) with 5% CO2. After 12 hours culture, the DMEM/F12 medium was completely replaced by neuro-basal medium (Hyclone, USA) which was added in 2% B27 (Hyclone, USA), 1% L-Glutamine (Hyclone, USA) and 1% penicillin-streptomycin solution (Hyclone, USA). Then, replaced half of the culture medium every 3 days.
After 14 days culture, the hippocampal primary neuronal were treated with 5 μM P7C3 (HY-15976, MCE, USA) for 2 hours [24,25], and then the neuronal were given 1.3% sevoflurane and 50% N2O continuous incubated for 4 hours (P7C3 group), and the control group mice with 50% O2. The Sev-N2O group was continuous incubated with 1.3% sevoflurane and 50% N2O, but no P7C3 advanced treated.
Flow cytometry detect apoptosis
This experiment requires at least 1×106 cells. The cells were washed with PBS for twice, and then added 80% ethanol overnight. The supernatant was removed by centrifugation (800 rpm for 10 minutes), washed with PBS and resuspend with PC buffer (300 μl, 1.611% NaHPO4.12H2O, 0.074% Sodium citrate, pH=7.8), standing for 30 minutes at room temperature. The following operation is carried out according to the instructions of Annexin V-FITC Apoptosis Detection Kit I (Abcam, UK). Barely, added 5 μl Annexin V-FITC to mix well and incubate at room temperature for 15 min. Then added 5 μl PI buffer at 5 minutes before the test. At last, added 200 μl PC buffer to detect.
Data analysis
All data was analyzed by SPSS20.0, T test or one-way analysis of variance was used to compare differences between groups in the data of behavioral scoring. P-value less than 0.05 was considered significant difference.
Results
Sev-N2O anesthesia induced short-term impairment of spatial learning and memory in aged rats
There was no hypoxia rats (SpO2<90%) during anesthesia, and no dyskinesia and floating rats during positioning sailing training. In first day, all rats tended to swim to the edge of the pool for escape. However, rats gradually learned the important of a platform for escape after a platform was placed at the end of each trial. In second day, all rats tended to swim to the platform for escape, and the latency time (time for locate the platform after being put into the pool) was significantly less than the first day (P<0.05). Repeat the measurement data of latency in two groups, and then analyze the data. The rats in anesthesia had a significantly longer latency time than control group in day 2 (P=0.003), day 3 (P=0.005), day 4 (P=0.035) and day 5 (P=0.042) (Figure 1A), but no significant difference on swimming speed during the trial (Figure 1B).
Figure 1.
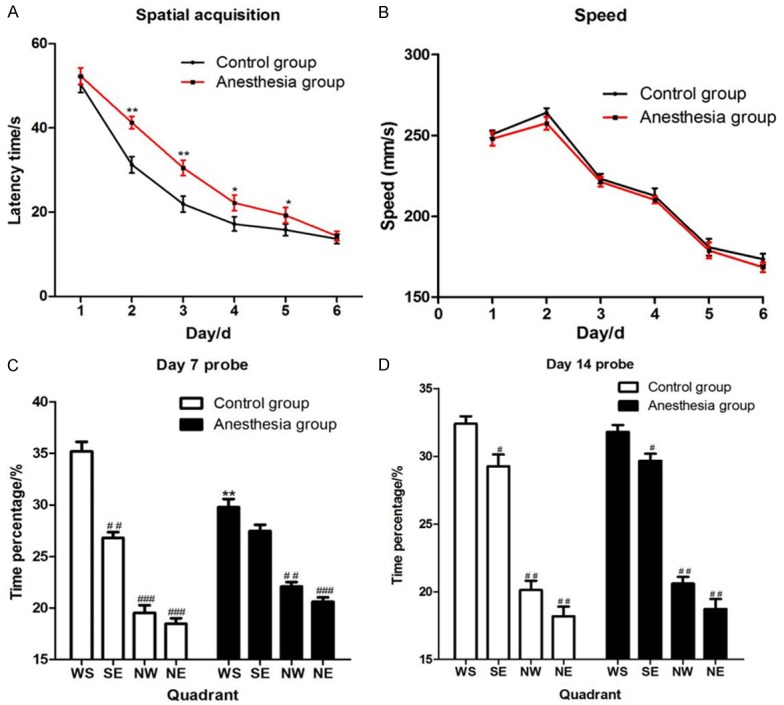
Data of positioning sailing training and probe test in Morris water maze test. A. The latency time of two group rats during positioning sailing training, and there was a significant difference in day 2 (P=0.003), day 3 (P=0.005), day 4 (P=0.035) and day 5 (P=0.042). * was P<0.05 and ** was P<0.001 which was compared with the control group. B. The swimming speed of two group rats during positioning sailing training, and had no significant difference. C, D. The time spent in target quadrant (WS quadrant) in control group was much more than the anesthesia group (P=0.002) in day 7 probe test, but no significant difference in day 14 probe test (P=0.079). ** was P<0.001 compared with the control group. # was P<0.05 and ## was P<0.01 compared with the time percentage of WS quadrant.
The first probe test conducted 24 hours after the last positioning sailing training was used to assess the memory of spatial. In the first probe test, the WS quadrant was the target quadrant, and the percentage of time spend in every quadrant was recorded. In Figure 1C, two groups rats all spent more time in target quadrant, but the control group rats spent significantly more than the anesthesia group (P=0.002). After 7 days of the first probe test, the second probe test had been conducted, and the control group rats did not spent significantly more than the anesthesia group (P=0.079) in target quadrant (Figure 1D).
Sev-N2O anesthesia induced neuronal apoptosis in rats
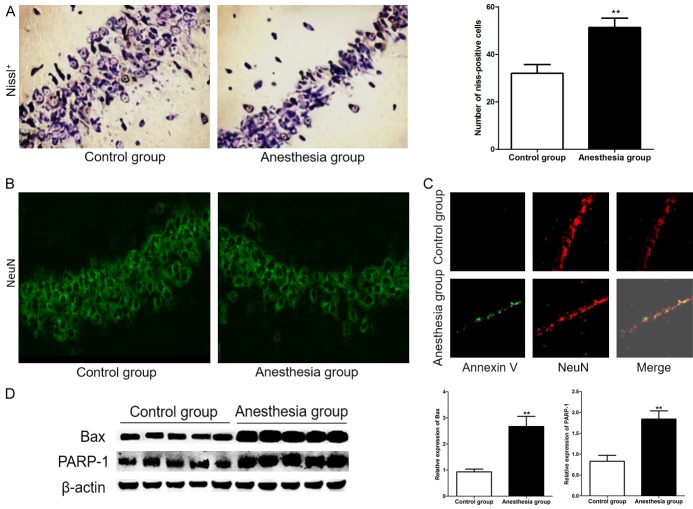
After 48 hours of Sev-N2O anesthesia, some rats in anesthesia group and control group were executed, and the hippocampus was removed for Nissl staining, staining with NeuN, doubled staining with Annexin V-FITC and NeuN, western Blot. As shown in Figure 2A, the number of Nissl positive cell in anesthesia group was significantly more than controls (32.00±3.71 vs 51.30±3.95, P=0.000) (Figure 2B). And the expression of the NeuN in CA1 subfield decreased in exposed rats compared with controls.
Figure 2.
Neuronal death increased in rats after 48 hours of Sev-N2O anesthesia. A. Nissl positive cells were significantly reduced in anesthesia group rats which were compared with control group. ** was P<0.01 compared with control group. B. Expression of the NeuN in CA1 subfield decreased in exposed rats compared with controls. C. More neurons apoptotic in CA1 area in Anesthesia group compared with controls, which was detected by doubled staining with Annexin V-FITC and NeuN. D. Western blot show that the expression of Bax and PARP-1 was significantly increased in Anesthesia group compared with controls. ** was P<0.01 compared with control group.
To confirm the neuronal loss was resulted from the neuronal apoptosis, we examined neuronal apoptosis by doubled staining with Annexin V-FITC and NeuN, and the result shown that the Annexin V positive neuronal was significant more in CA1 of anesthesia group rats than in controls. But most of the Annexin V-positive cells also stained positive for NeuN (Figure 2C).
To study the molecular mechanism of neuronal apoptosis, the expression of Bax and PARP-1 protein was detected by western blot. In Figure 2D, we saw that the expression of Bax and PARP-1 protein was significantly increased compared with controls (P<0.001).
Sev-N2O anesthesia induced Nampt and RelB protein decrease in rats
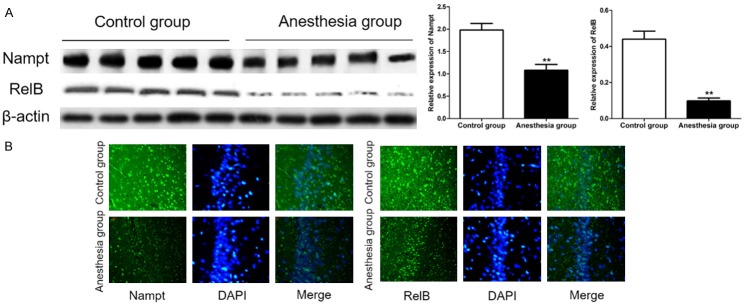
Western blot and immunofluorescence were used to detect the expression of Nampt and RelB in hippocampus of two groupsof rats after 48 hours of Sev-N2O anesthesia. As shown in Figure 3, the expression of Nampt and RelB in hippocampus of anesthesia group was significantly decreased compared with controls (P<0.01).
Figure 3.
Sev-N2O anesthesia induced Nampt and RelB protein decrease in rats. A. The expression of Nampt and RelB protein was detected by immunoblotting. ** was P<0.01 compared with control group. B. Nampt positive cells and RelB positive cells were decreased compared with controls.
Nampt activator P7C3 attenuated Sev-N2O anesthesia induced hippocampal primary neuronal apoptosis
AS shown in Figure 4A, Apoptotic hippocampal primary neurons were significantly higher after Sev-N2O anesthesia, but P7C3 stimulation in advance could attenuate apoptosis. And we detected the expression of Nampt, RelB, Bax, PARP-1 protein by western blot, it was shown that Nampt and RelB protein were up-regulated, and Bax, PARP-1 protein were down-regulated by P7C3 stimulation in advance compared with Sev-N2O group.
Figure 4.
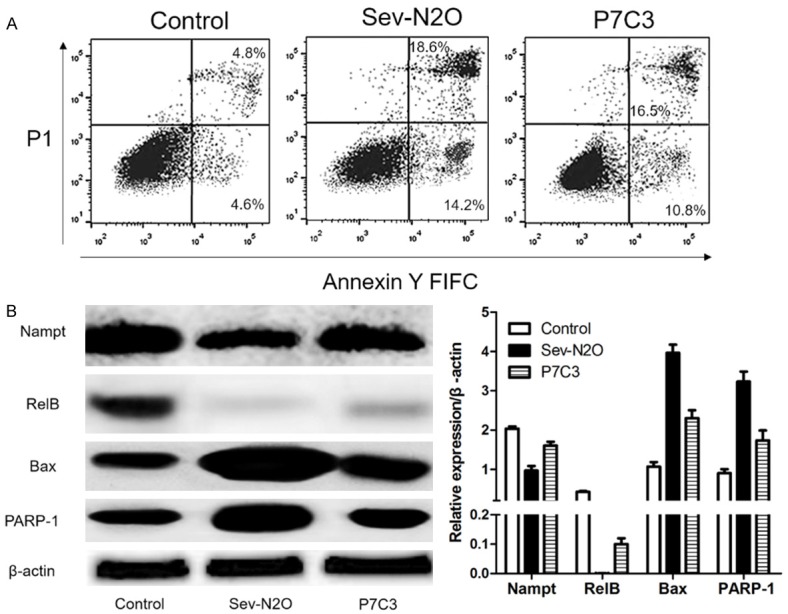
Expression of Nampt, RelB, Bax, PARP-1 was detected by western blot and hippocampal primary neuronal apoptosis by Flow cytometry. A. P7C3 attenuated Sev-N2O anesthesia induced hippocampal primary neuronal apoptosis. B. Nampt and RelB protein were up-regulated, and Bax, PARP-1 proteins were down-regulated by P7C3 stimulation in advance compared with Sev-N2O group.
Discussion
POCD could occur in different age, and it is not conducive to early postoperative rehabilitation and long-term quality of life of patients, and advanced age is currently the only widely recognized risk factor for long-term or irreversible POCD [26,27]. With the aging of the age, social adaptability will be a progressive loss. When a life accident or traumatic event occurs, changes in the environment require the body to be more adaptable, the need and abilities are out of balance and lead to memory loss in the elderly [28].
In addition, β-amyloid naturally exists in the central nervous system, and in the elderly brain its concentration was significantly higher than in adults. Therefore, after general anesthesia for elderly patients, it can increase the risk of the occurrence and development of POCD by directly influencing the aggregation rate of Aβ in the brain [29]. In the elderly, the brain is different from young people in terms of volume, distribution, neurotransmitter type, metabolic function, and plasticity, which can be more sensitive to the anesthesia-mediated neurotoxicity [30]. Based on the above reasons, we use 18-month-old rats as the object to explore the effects and potential mechanisms on cognitive function after inhalation anesthesia.
In the present study, we found that Sev-N2O anesthesia induced a significant deficit in short-term spatial learning acquisition and memory retention, but it had no significant deficit in long-term. As early as 1955, Beford et al. had suggested that the occurrence of cognitive dysfunction was associated with narcotic drugs [31]. Although inhaled anesthetics can be removed from the body very quickly, it may still lead to changes in brain morphology and function over time.
Recently, a randomized controlled trial by Meineke et al. showed that elderly patients (≥65 years) were given sevoflurane or diflurane inhaled anesthesia and maintained appropriate anesthesia depth, and both groups experienced a brief decline in cognitive function after anesthesia [32]. And a large number of animal experiments also suggested that isoflurane, N2O, isoflurane combined with nitrous oxide and sevoflurane have neurotoxic effected on both adult and elderly brains, and eventually lead to impaired cognitive function [33-37]. A study by Wikland et al. showed that mice had memory dysfunction after 2 hours of inhalation of 2.6% sevoflurane, and rats showed dose-dependent memory impairment after 2 hours of exposing to 0.5%, 1.0% and 2% sevoflurane [38,39]. Peng et al. also showed that the expression of IGF-1 in elderly rats can be reduced after 2 hours of inhalation of 3% seflurane, and its cognitive function is also affected [40].
Although many studies have shown that inhaled anesthetics can cause postoperative cognitive impairment, there are also some arguments raised by other researchers [41,42]. Callaway et al. showed that young adult rats and elderly rats (22-24 months) after 4 hours of exposing to sevoflurane, 1 week later, the behavioral experiment suggesting that exposing to sevoflurane promote the acquisition of learning, and there was no significant difference in the extraction of spatial memory between the intervention group of adult rats or elderly rats and the corresponding control group [43]. The results of this contradiction are not yet reasonably explained, but it may be related to the type of drug used, dosage, duration of exposure and gas carrier, in addition to the animal species, age, and rearing environment.
Although we cannot explain the opposite findings from different studies, we can confirm that narcotic drugs-induced neuronal apoptosis play an important role in the pathogenesis of POCD [8,9]. Therefore, we detected the apoptosis of neurons in hippocampus of rats 48 hours after Sev-N2O anesthesia. The result shown that the neuronal apoptosis and the expression of Bax, PARP-1 in hippocampus of rats increased significantly after Sev-N2O anesthesia. It was indicated that Sev-N2O anesthesia may cause induce short-term POCD by promoting the apoptosis of neuronal cells in the hippocampus of rats in the present study.
Nampt is the rate-limiting enzyme for mammalian NAD+ remediation synthesis pathway, and it has two functions. Firstly, Nampt could affect material synthesis and energy metabolism, protein modification, DNA repair and other important processes in cell [44]. Secondly, Nampt regulates the inflammatory response in vivo as an anti-inflammatory factor [45,46]. Recently, lots of studies suggest that Nampt is associated with aging and aging-related diseases, and have presented the Nampt-NAD-sirtuin aging hypothesis [18,47-50]. As a Rate limiting enzyme of NAD and a coenzyme of PARP-1, Nampt could protect neurons [48], inhibits apoptosis [49] and axonal degeneration [50]. Moreover, Nampt-SIRT1-Relb can inhibit the high inflammatory response in vivo [45,46].
In the present study, the expression of Nampt, RelB decreased significantly 48 hours after Sev-N2O anesthesia, and Nampt activators could reduce the apoptosis of hippocampal primary cells in vitro after 4 hours exposed with Sev-N2O bypromoting the expression of Nampt/RelB. In the lower organisms such as yeast, the longevity gene PNC1 can affect the synthesis of NAD by synthesizing nicotinic acid, and activate Sir2 protein, to extend the life of the function. NAMPT plays a similar function to PNC1 in mammal. It could fight aging by synthesizing nicotinamide mononucleotide for NAD [48]. In addition, the expression of Nampt is also associated with the development of neurodegenerative diseases. Nampt is widely distributed in neurons, and it is helpful to delay the development of neurodegenerative diseases by up-regulating the expression of Nampt, while down-regulating the expression of Nampt will induce cognitive impairment [20-22,51].
In conclusion, down-regulation of Nampt/RelB signaling was closely related to neuronal apoptosis in the hippocampus contributed to the neurotoxicity and cognitive dysfunction induced by general anesthesia with sevoflurane-nitrous oxide, and the mechanism may be related to the down-regulation of Nampt/RelB expression in promoting hippocampal neuronal apoptosis.
Acknowledgements
The research was supported by Beijing Municipal Natural Science Foundation (8072015).
Disclosure of conflict of interest
None.
References
- 1.Sawamura S. Postoperative cognitive dysfunction. Bja British Journal of Anaesthesia. 2012;172:82–87. doi: 10.1093/bja/aei062. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Rappold T, Laflam A, Hori D, Brown C, Brandt J, Mintz CD, Sieber F, Gottschalk A, Yenokyan G, Everett A, Hogue CW. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery. Br J Anaesth. 2016;116:83–9. doi: 10.1093/bja/aev415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross AF, Stern TA. Neuropsychiatric conditions associated with anesthesia exposure. Psychosomatics. 2014;55:21–28. doi: 10.1016/j.psym.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Jungwirth B, Zieglgänsberger W, Kochs E, Rammes G. Anesthesia and postoperative cognitive dysfunction (POCD) Mini Rev Med Chem. 2009;9:1568–79. doi: 10.2174/138955709791012229. [DOI] [PubMed] [Google Scholar]
- 6.Hansen MV. Chronobiology, cognitive function and depressive symptoms in surgical patients. Dan Med J. 2014;61:B4914. [PubMed] [Google Scholar]
- 7.Zhang Z, Yuan H, Zhao H, Qi B, Li F, An L. PPARγactivation ameliorates postoperative cognitive decline probably through suppressing hippocampal neuroinflammation in aged mice. IntImmunopharmacol. 2017;43:53–61. doi: 10.1016/j.intimp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 8.van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67:280–293. doi: 10.1111/j.1365-2044.2011.07008.x. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology. 2007;106:622–628. doi: 10.1097/00000542-200703000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Byrne RP. Review of inhalation anesthesia. Journal of Oral & Maxillofacial Surgery. 2007;65:29–29. [Google Scholar]
- 11.Jadhav P. A comparison of desflurane and sevoflurane in the recovery of cognitive function after general anesthesia in elderly patients. Journal of Applied Polymer Science. 2017;120:3278–3282. [Google Scholar]
- 12.Zhang W, Zhang L, Li H, et al. GC-MS based serum metabolomic analysis of isoflurane-induced postoperative cognitive dysfunctional rats: biomarker screening and insight into possible pathogenesis. Chromatographia. 2012;75:799–808. [Google Scholar]
- 13.Liu Y, Sun LY, Singer DV, Ginnan R, Singer HA. CaMKII-dependent inhibition of cAMP-response element-binding protein activity in vascular smooth muscle. J Biol Chem. 2013;288:33519–29. doi: 10.1074/jbc.M113.490870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang F, Lin W, Ling X, Song R, Liu Q, Lai B, Cang J. The hippocampal cyclin D1 expression is involved in postoperative cognitive dysfunction after sevoflurane exposure in aged mice. Life Sci. 2016;160:34–40. doi: 10.1016/j.lfs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Xie H, She GM, Wang C, Zhang LY, Liu CF. The gender difference in effect of sevoflurane exposure on cognitive function and hippocampus neuronal apoptosis in rats. Eur Rev Med Pharmacol Sci. 2015;19:647–57. [PubMed] [Google Scholar]
- 16.Le FH, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N, Buée-Scherrer V, Lebuffe G, Blum D, Buée L. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116:779–787. doi: 10.1097/ALN.0b013e31824be8c7. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Gong M, Yan M, Zhang X. Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PLoS One. 2013;8:e57870. doi: 10.1371/journal.pone.0057870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai S. From heterochromatin islands to the NAD world: a hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim Biophys Acta. 2009;1790:997–1004. doi: 10.1016/j.bbagen.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi M, Yamamoto T. Apoptosis induced by NAD depletion is inhibited by KN-93 in a CaMKII-independent manner. Exp Cell Res. 2015;335:62–7. doi: 10.1016/j.yexcr.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Hong Y, Nie H, Wei X, Fu S, Ying W. NAD(+) treatment can prevent rotenone-induced increases in DNA damage, bax levels and nuclear translocation of apoptosis-inducing factor in differentiated PC12 cells. Neurochem Res. 2015;40:837–42. doi: 10.1007/s11064-015-1534-0. [DOI] [PubMed] [Google Scholar]
- 21.Villela D, Schlesinger D, Suemoto CK, Grinberg LT, Rosenberg C. A microdeletion in Alzheimer’s disease disrupts NAMPT, gene. J Genet. 2014;93:535–7. doi: 10.1007/s12041-014-0399-3. [DOI] [PubMed] [Google Scholar]
- 22.Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, Zorumski CF, Imai S. Expression of nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J Neurosci. 2014;34:5800–5815. doi: 10.1523/JNEUROSCI.4730-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, Swerdlow RH, Wang X, Ding S. Deletion of nampt in projection neurons of adult mice leads to motor dysfunction, neurodegeneration, and death. Cell Rep. 2017;20:2184–2200. doi: 10.1016/j.celrep.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–34. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, Kotti T, Ure K, Brat DJ, Williams NS, MacMillan KS, Naidoo J, Melito L, Hsieh J, De Brabander J, Ready JM, McKnight SL. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perouansky M. General anesthetics and longterm neurotoxicity. Handb Exp Pharmacol. 2008;182:143–157. doi: 10.1007/978-3-540-74806-9_7. [DOI] [PubMed] [Google Scholar]
- 27.Perouansky M. Liaisons dangereuses? General anaesthetics and long-term toxicity in the CNS. Eur J Anaesthesiol. 2007;24:107–115. doi: 10.1017/S0265021506001165. [DOI] [PubMed] [Google Scholar]
- 28.Charles E, Boubyserieys V, Thomas P, Clément JP. [Links between life events, traumatism and dementia; an open study including 565 patients with dementia] . Lencéphale. 2006;32:746–52. doi: 10.1016/s0013-7006(06)76227-3. [DOI] [PubMed] [Google Scholar]
- 29.Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB. Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth. 2006;97:445–52. doi: 10.1093/bja/ael233. [DOI] [PubMed] [Google Scholar]
- 30.Naudí A, Cabré R, Jové M, Ayala V, Gonzalo H, Portero-Otín M, Ferrer I, Pamplona R. Chapter five-lipidomics of human brain aging and alzheimer’s disease pathology. Int Rev Neurobiol. 2015;122:133–189. doi: 10.1016/bs.irn.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–63. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- 32.Meineke M, Applegate RL 2nd, Rasmussen T, Anderson D, Azer S, Mehdizadeh A, Kim A, Allard M. Cognitive dysfunction following desflurane versus sevoflurane general anesthesia in elderly patients: a randomized controlled trial. Med Gas Res. 2014;4:1–9. doi: 10.1186/2045-9912-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- 34.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–14. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Culley DJ, Raghavan SV, Waly M, Baxter MG, Yukhananov R, Deth RC, Crosby G. Nitrous oxide decreases cortical methionine synthase transiently but produces lasting memory impairment in aged rats. Anesth Analg. 2007;105:83–8. doi: 10.1213/01.ane.0000266491.53318.20. [DOI] [PubMed] [Google Scholar]
- 36.Mawhinney LJ, de Rivero Vaccari JP, Alonso OF, Jimenez CA, Furones C, Moreno WJ, Lewis MC, Dietrich WD, Bramlett HM. Isoflurane/nitrous oxide anesthesia induces increases in NMDA receptor subunit NR2B protein expression in the aged rat brain. Brain Res. 2012;1431:23–34. doi: 10.1016/j.brainres.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 38.Wiklund A, Granon S, Faure P, Sundman E, Changeux JP, Eriksson LI. Object memory in young and aged mice after sevofluraneanaesthesia. Neuroreport. 2009;20:1419–23. doi: 10.1097/WNR.0b013e328330cd2b. [DOI] [PubMed] [Google Scholar]
- 39.Liu XS, Xue QS, Zeng QW, Li Q, Liu J, Feng XM, Yu BW. Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3β in the hippocampus. Neurobiol Learn Mem. 2010;94:461–467. doi: 10.1016/j.nlm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Peng S, Zhang Y, Sun DP, Zhang DX, Fang Q, Li GJ. The effect of sevoflurane anesthesia on cognitive function and the expression of insulin-like growth factor-1 in CA1 region of hippocampus in old rats. MolBiol Rep. 2011;38:1195–9. doi: 10.1007/s11033-010-0217-9. [DOI] [PubMed] [Google Scholar]
- 41.Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgänsberger W, Ohl F, Kochs EF, Blobner M. Isofluraneanaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–36. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Haseneder R, Starker L, Berkmann J, Kellermann K, Jungwirth B, Blobner M, Eder M, Kochs E, Rammes G. Sevoflurane anesthesia improves cognitive performance in mice, but does not influence in vitro long-term potentation in hippocampus CA1 stratum radiatum. PLoS One. 2013;8:e64732. doi: 10.1371/journal.pone.0064732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callaway JK, Jones NC, Royse AG, Royse CF. Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology. 2012;117:1091–101. doi: 10.1097/ALN.0b013e31826cb228. [DOI] [PubMed] [Google Scholar]
- 44.Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem. 2011;286:9856–64. doi: 10.1074/jbc.M110.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang P, Du H, Zhou CC, Song J, Liu X, Cao X, Mehta JL, Shi Y, Su DF, Miao CY. Intracellular NAMPT-NAD+-SIRT1 cascade improves postischaemic vascular repair by modulating notch signalling in endothelial progenitors. Cardiovasc Res. 2014;104:477–88. doi: 10.1093/cvr/cvu220. [DOI] [PubMed] [Google Scholar]
- 47.Imai S. A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res. 2010;62:42–7. doi: 10.1016/j.phrs.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1962–71. doi: 10.1038/jcbfm.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–5. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, He Z. NAD and axon degeneration: from the Wldsgene to neurochemistry. Cell AdhMigr. 2009;3:77–87. doi: 10.4161/cam.3.1.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]