Abstract
Chemokine (C-X-C motif) ligand (CXCL) is a class of secreted growth factor that signals through a G-protein coupled receptor. CXCL protein family members play important roles in inflammation and aberrant expression is associated with growth and progression of certain tumors. To explore the expression pattern and action mechanism of CXCL1 and CXCL8 in development of gastric carcinoma (GC), 72 cases of GC and para-carcinoma tissue specimens were used for experimental study, and qPCR was used for analysis on the expression of CXCL1 and CXCL8 in GC specimens. For in vitro culture of GC cell HGC27, knockout of CXCL1 and CXCL8 genes for GC cell HGC27 was performed through RNA interference, proliferation of HGC27 cells was tested by MTT, apoptosis of HGC27 cells was tested by flow cytometry, and the influence of CXCL1 and CXCL8 on HGC27 cell migration was tested by transwell. CXCL1 and CXCL8 expression level in HGC27 cells was analyzed by Western blotting. Co-immunoprecipitation (co-IP) was used for identifying interaction of CXCL1 and CXCL8 with CXCR2 in GC cells. The results show that both CXCL1 and CXCL8 expression were significantly up-regulated. Relevant clinical data showed that low expression of CXCL1 and CXCL8 significantly correlated with features for poor prognosis of GC, including serum alpha-fetoprotein (AFP) level, tumor size, and TNM staging. Down-regulation of CXCL1 and CXCL8 may up-regulate expression of each other and thus silencing expression of CXCL1 and CXCL8 may significantly inhibit proliferation and migration capabilities of HGC27 cells, and induce the apoptosis. Downregulated CXCL1 and CXCL8 expression in GC cells may significantly intensify interaction of one another and with CXCR2. The above results indicate that CXCL1 and CXCL8 participate in GC proliferation, apoptosis, and migration processes through specific binding with CXCR2 by a synergistic effect.
Keywords: CXCL1, CXCL8, gastric carcinoma, CXCR2, prognosis
Introduction
Gastric carcinoma (GC) is the fourth most common malignant tumor worldwide. Although the incidence rate of GC greatly decreased globally in recent past decades, most GC patients have been diagnosed in middle or advanced stage, which was extremely unfavorable for treatment [1]. In recent years, molecularly targeted therapy has gradually become a hot topic for research on cancer therapy. Growth, proliferation, and metastasis of tumor cells are a sustaining process, which need a continuous energy supply, and angiogenesis provides a material basis for the process [2-4]. Chemokines control directional migration of cells [5] and if the expression and function of the chemokine and its receptor are abnormal, immunocytes will not be able to function properly at correct positions [6]. The chemokines of CXCL1 and CXCL8 play an important role in pathogenic processes for several cancers [7,8]. Relevant studies found that CXCL1 and CXCL8 could promote angiogenesis [9,10]. Researchers using genetically engineered mice have proven that angiogenesis has a promoting effect via CXCL1. Their studies showed that CXCL1 could induce angiogenesis through absorbing neutrophils and causing secretion of vascular endothelial growth factors by neutrophils [11]. CXCL8 is a key factor for promoting growth and metastasis of GC, as well as angiogenesis [12]. Some studies showed that CXCL8 could adjust tumor cells and peripheral matrix cells into secreting matrix metalloproteinases MMP-2 and MMP-9, and which strengthens their activities. MMP-2 and MMP-9 could advance the activity of perivascular collagenase, as well as the degradation of peripheral matrix, and promote the movement of endothelial cells to the direction of the angiogenesis factor along a degraded extracellular matrix, thereby promoting formation of tumor vessels [13].
However, signaling pathways upstream of CXCL1 and CXCL8 regulate expression. NF-κB plays an important role in expression regulation and control of CXCL1 and CXCL8. NF-κB can be activated by Bcl-2 and the activated NF-κB can up-regulate expression of CXCL1 and CXCL8 in vascular endothelial cells and induce migration of cancer cells [14]. The consistent effect of CXCL1 and CXCL8 in tumor vessel formation made us wonder whether there is a supplementary action between CXCL1 and CXCL8 in GC occurrence and development processes. The mechanism for regulation of the growth of GC is still unknown. In this study, to clarify the action mechanism of CXCL1 and CXCL8 in the pathogenetic process of GC, we measured the expression level of CXCL1 and CXCL8 in 72 cases of GC tissues and analyzed the correlation of CXCL1 or CXCL8 expression with the pathogenesis of GC. Using RNA interference for knockout of CXCL1 and CXCL8 genes in GC cell HGC27 we also tested the influence of CXCL1 and CXCL8 gene knockout on proliferation, migration, and apoptosis.
Materials and methods
Tissue collection
Fresh frozen GC and adjacent tissues were obtained from 72 patients who were diagnosed with GC and underwent radical gastrectomy at the department of Gastroenterology and Hepatology, The First Affiliated Hospital of Wenzhou Medical University from January 2009 to December 2011. None of these patients underwent local or systemic therapy before surgery. This research was approved by the Research Ethics Committee of Wenzhou Medical University, China. And all patients signed the written informed consent.
Quantitative real-time PCR
Total RNA was extracted and isolated from GC tissue samples using Trizol reagent (Invitrogen, USA) following the user’s manual. SYBR PremixExTaq™ (Takara, Japan) was used in Quantitative real-time PCR (qRT-PCR) on BIO-RAD MY IQ (USA). All primer sequences are shown in Table 1. The relative expression of CXCL1 and CXCL8 was shown as fold difference relative to GAPDH.
Table 1.
Primer sequences of expression products
| Gene name | Primer name | Primer sequence |
|---|---|---|
| CXCL1 | Forward primer | 5’CGCTACAGCGACGTGAAGAA3’ |
| Reverse primer | 5’GTTCCAGGCGTTGTACCAC3’ | |
| CXCL8 | Forward primer | 5’CTTTGTCCATTCCCACTTCTGA3’ |
| Reverse primer | 5’TCCCTAACGGTTGCCTTTGTAT3’ | |
| GAPDH | Forward primer | 5’ATGTCGTGGAGTCTACTGGC3’ |
| Reverse primer | 5’TGACCTTGCCCACAGCCTTG3’ |
Cell culture conditions and transfection
The human GC cell lines HGC27 were purchased from American Type Culture Collection (ATCC, USA), and the normal gastric mucosal epithelial cell line GES-1 was purchased from CICLR (Beijing, China). Cells were cultured in (RPMI) 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin (Sigma, USA), at 37°C, in 5% CO2 incubator (Memmert, Germany). The siRNA sequence of CXCL1 and CXCL8 genes were designed by using Invitrogen (an online software), and the siRNA for CXCL1 and CXCL8 were purchased from Ribobio (Ribobio, China). The human GC cell lines HGC27 were transfected with siRNAs with Lipofectamine 2000 (Invitrogen) following the user manual. Western Blot was used to verify the transfection effect.
Cell proliferation assays
Cell Proliferation Reagent Kit I was used to detect the proliferation of cells that were transfected by CXCL1 and CXCL8 siRNA. After transfection of CXCL1 and CXCL8 siRNA for 48 h, the cells were transferred to a 96-hole cell culture plate at a concentration of 5,000 cells per well. After 24 hours, cell proliferation was recorded and the data were analyzed according to instructions provided by the kit. Each experiment was repeated 3 times.
Flow-cytometric assays
After transfection of CXCL1 and CXCL8 siRNA for 48 h, cells were collected by trypsin digestion. After resuspension, the cell were seeded at a concentration of 105 cells/ml. Cell apoptosis rate was detected by flow cytometry after double staining with FITC and PI. According to the characteristics of the cell, the cells were divided into living cells, dead cells, early apoptotic cells and late apoptotic cells. The proportion of apoptotic cells was calculated by the flow cytometry software. All samples were tested three times.
Transwell assays
HGC27 cells were resuspended 48 h after transfection, and then trypan blue staining was performed and the cell survival rate was determined. If the rate exceeded 95%, then follow-up experiments were conducted. The experimental operation was performed strictly in compliance with the instructions for use. The cells were inoculated in the upper chambers of the transwell with or without matrigel, at a density of 106/well, which was respectively used for determining migration and invasion abilities of HGC27 cells; for the invasion test, a medium containing 10% fetal calf serum was added in the lower chambers of transwell. The cells were placed in an incubator for culture, and 4% paraformaldehyde fixation was performed at 20 min, 12 h, and 24 h after the culture. Eosin staining was done at 30 min, together with step-by-step dehydration based on an ethanol gradient. This was followed by dimethylbenzene transparentizing and neutral balsam mounting. An inverted microscope (Olympus, Japan) was used for imaging and the cells under each field were counted. For each group, 3 batches of samples were counted.
Co-IP assays
In combination with the results of proliferation, apoptosis, and transwell testing, we proposed that CXCL1 and CXCL8 may participate in development of GC through a receptor CXCR2 competition. Therefore, we designed a co-immunoprecipitation assay for determining the correlation of CXCL1 and CXCL8 with ligand CXCR2. For HGC27 cells, after transfection of 48 h, the medium was discarded, cells were scraped, rinsed twice with cold PBS solution, and centrifugal separation was performed. The supernatant was discarded and 100 μl of RIPA was added on an ice bath for 30 min, followed by primary antibody, rotational mixing at 4°C for 2 h, addition of 20 μg of protein A, and continued mixing for 12 h, followed by centrifugal separation, washing with RIPA for three times, adding 20 μl 2x Tricine SDS-PAGE loading buffer, boiling for 5 min, and then electrophoresis.
Western blot assays
For the collected and treated cells, total cellular protein was extracted by RIPA lysis, and electrophoresis of samples was done with 10% SDS-PAGE. 20 μl per well was loaded, after which transmembrane electroporation was done followed by blocking for 1 h with 5% skim milk powder. Diluted rabbit-anti CXCR2 (1:1000), CXCL1 (1:2000) and CXCL8 (1:1000) antibodies were then added for overnight incubation at 4°C, washing 3 times with 0.1% TBST, 5 min/time, goat anti-rabbit IgG of dilution with a ratio of 1:10000, incubation at room temperature for 30 min, washing the membrane, adding substrate solution for chemiluminescence, developing, and analyzing the optical density of the bands by ImageJ software.
Statistical analysis
Data are presented as mean ± SEM. A Pearson chi-squared test was applied to determine clinicopathological correlations. The Kaplan-Meier method was used to calculate the survival curves. Significance was determined by the log-rank test. The difference among groups was determined by ANOVA analysis and comparison between two groups was analyzed by the Student’s t-test using GraphPad software version 5.0 (GraphPad Software, CA). Difference were considered significant when P < 0.05.
Results
Up-regulation of CXCL1 and CXCL8 in GC tissues
With para-carcinoma tissue as the control, the CXCL1 and CXCL8 expression levels in 72 cases of GC tissues were tested with qRT-PCR. The expression levels of CXCL1 and CXCL8 in GC tissues increased significantly as compared with in para-carcinoma tissues (Figure 1A, 1B). Based on the median of expression levels, the subjects were divided into a high expression group and low expression group. As shown in Tables 2 and 3, among GC patients, the higher the CXCL1 and CXCL8 expression, the higher the AFP level, the bigger the tumor volume, and the higher the TNM tumor stage.
Figure 1.
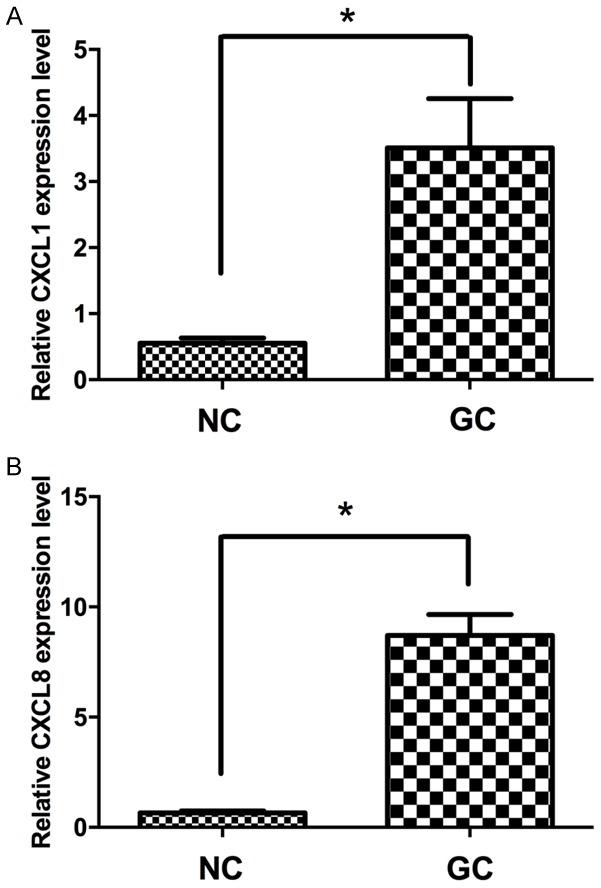
The relative expression levels of CXCL1 and CXCL8 in GC tissues. CXCL1 (A) and CXCL8 (B) were detected in 72 pairs of GC tissues with qRT-PCR. Data are presented as fold change in tumor tissues relative to normal tissues. NC, normal control tissues, GC, GC tissues. *P < 0.05.
Table 2.
Correlation of the expression of CXCL1 with clinicopathologic features
| Clinicopathologic features | n (%) | Relative expression of CXCL1 | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age (year) | ||||
| < 50 | 32 (44.4%) | 15 | 17 | 0.963 |
| ≥50 | 40 (55.6%) | 21 | 19 | |
| Sex | ||||
| Male | 39 (54.2%) | 15 | 24 | 0.632 |
| Female | 33 (45.8%) | 13 | 20 | |
| Serum AFP level (ng/mL) | ||||
| < 20 | 14 (19.4%) | 2 | 12 | 0.007** |
| ≥20 | 58 (80.6%) | 40 | 18 | |
| Tumor size (cm) | ||||
| < 5 | 26 (36.1%) | 8 | 18 | 0.025* |
| ≥5 | 46 (63.9%) | 35 | 11 | |
| TNM tumor stage | ||||
| I+II | 51 (70.8%) | 14 | 37 | 0.019* |
| III+IV | 21 (29.2%) | 17 | 4 | |
Compared with low CXCL1 expression group;
P < 0.05;
P < 0.01.
Table 3.
Correlation of the expression of CXCL8 with clinicopathologic features
| Clinicopathologic features | n (%) | Relative expression of CXCL8 | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age (year) | ||||
| < 50 | 32 (44.4%) | 19 | 13 | 0.997 |
| ≥50 | 40 (55.6%) | 22 | 18 | |
| Sex | ||||
| Male | 39 (54.2%) | 20 | 19 | 0.946 |
| Female | 33 (45.8%) | 18 | 15 | |
| Serum AFP level (ng/mL) | ||||
| < 20 | 14 (19.4%) | 5 | 9 | 0.008** |
| ≥20 | 58 (80.6%) | 46 | 12 | |
| Tumor size (cm) | ||||
| < 5 | 26 (36.1%) | 5 | 21 | 0.009** |
| ≥5 | 46 (63.9%) | 39 | 7 | |
| TNM tumor stage | ||||
| I+II | 51 (70.8%) | 15 | 36 | 0.023* |
| III+IV | 21 (29.2%) | 18 | 3 | |
Compared with low CXCL8 expression group;
P < 0.05;
P < 0.01.
Negative correlation of CXCL1 and CXCL8 expression with survival time
To explore the correlation of CXCL1 and CXCL8 expression with GC prognosis, we conducted a Kaplan-Meier survival analysis on 72 patients. The results showed that the higher the CXCL1 and CXCL8 expression quantity, the shorter the survival time of the patient. The data showed that CXCL1 and CXCL8 expression quantity played an important role in the process for emergence and development of GC (Figure 2A, 2B).
Figure 2.
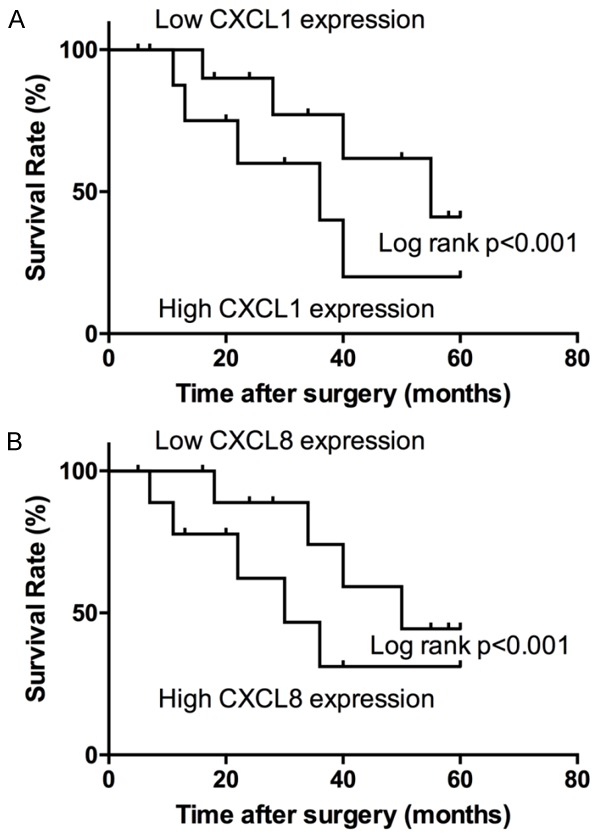
The survival analysis on 72 GC patients. Patients with low CXCL1 (A) and CXCL8 (B) expression showed increased survival times compared with patients with high CXCL1 and CXCL8 expression (P < 0.001, log-rank test).
CXCL1 and CXCL8 can promote proliferation of HGC27 cells
To explore the action mechanism of CXCL1 and CXCL8 in the GC pathogenesis, we performed knockout respectively for CXCL1 and CXCL8 through siRNA interference technology, and detected CXCL1 and CXCL8 expression in HGC27 cells after siRNA treatment through Western blot assay. The results showed that the siRNA experimental design can effectively down-regulate CXCL1 and CXCL8 expression (Figure 3A, 3B).
Figure 3.
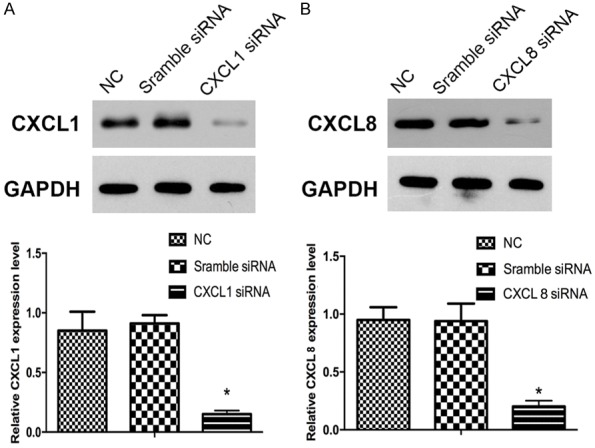
The proteins CXCL1 and CXCL8 were detected by Western blot after siRNA treatment in HGC27 cells. A. The expression of CXCL1 was knockdown with CXCL1- siRNA. B. The expression of CXCL8 was knockdown with CXCL8-siRNA. GAPDH was used as a control. Experiments were performed in triplicate. *P < 0.05, compared with control.
HGC27 cell proliferation was detected by MTT assay and the experimental results showed that after CXCL1 and CXCL8 expression was down-regulated, HGC27 cell proliferation was significantly inhibited (Figure 4). However, as compared with pure CXCL1 or CXCL8 down-regulation, down-regulation of both CXCL1 and CXCL8 had a more significant inhibitory effect on HGC27 cell proliferation, indicating that CXCL1 and CXCL8 could promote HGC27 cell proliferation through a synergistic effect.
Figure 4.
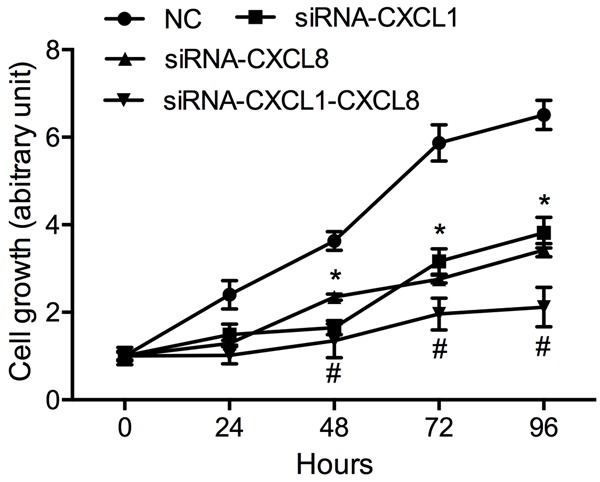
The effect of CXCL1 and CXCL8 on HGC27 cells proliferation. MTT assays were performed to determine the proliferation in HGC27 cells. Compared with NC, *P < 0.05. Compared with siRNA-CXCL1 or siRNA-CXCL8, #P < 0.05. Experiments were performed in triplicate. Data represent the mean ± S.D. from three independent experiments.
CXCL1 and CXCL8 can inhibit HGC27 cell apoptosis
Decrease cell apoptosis is an important feature in tumor development. After knockout for CXCL1 and CXCL8 through siRNA interference technology, we detected HGC27 cell apoptosis again. The experiment showed that down-regulation of CXCL1 and CXCL8 expression significantly promoted HGC27 cell apoptosis (Figure 5). However, as compared with pure CXCL1 or CXCL8 down-regulation, down-regulation of both CXCL1 and CXCL8 had a more significant promotion effect on HGC27 cell apoptosis, which indicated that CXCL1 and CXCL8 could induce HGC27 cell apoptosis through a synergistic effect.
Figure 5.

Apoptotic rates were detected by flow cytometry after siRNA treatment in HGC27 cells. UL, necrotic cells; UR, terminal apoptotic cells; LR, early apoptotic cells. Compared with NC, *P < 0.05. Compared with siCXCL1 or siCXCL8, #P < 0.05. Experiments were performed in triplicate. Data represent the mean ± S.D. from three independent experiments.
CXCL1 and CXCL8 can advance HGC27 cell migration and invasion
In cancer development, tumor cell migration is an important phase for middle or advanced stages. After CXCL1 and CXCL8 expression in HGC27 cells was inhibited, we tested cell migration and invasion. Transwell migration results showed that after down-regulation of CXCL1 and CXCL8 expression, HGC27 cell migration was significantly inhibited. However, as compared with pure CXCL1 or CXCL8 down-regulation, down-regulation of both CXCL1 and CXCL8 had a more significant inhibitory effect on HGC27 cell migration (Figure 6). Transwell invasion experiments showed that down-regulation of CXCL1 and CXCL8 expression significantly inhibited HGC27 cell invasion, which indicated that CXCL1 and CXCL8 could advance HGC27 cell migration and invasion through a synergistic effect.
Figure 6.
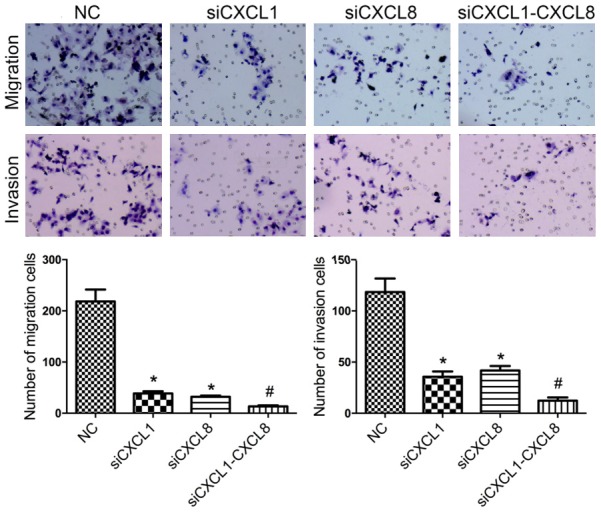
The effect of CXCL1 and CXCL8 on HGC27 cells migration and invasion. Compared with NC, *P < 0.05. Compared with siRNA-CXCL1 or siRNA-CXCL8, #P < 0.05. Experiments were performed in triplicate. Data represent the mean ± S.D. from three independent experiments.
Both CXCL1 and CXCL8 may be taken as the CXCR2 ligand
The results of co-IP assay showed that protein precipitated by CXCL1 antibodies resulted in detection of CXCR2 expression, but CXCL8 expression could not detected, whereas in the protein precipitated by CXCL8 antibodies, CXCR2 expression could be detected, but CXCL1 expression could not detected (Figure 7A). However, in the protein precipitated by CXCR2 antibodies both CXCL1 expression and CXCL8 expression could be detected (Figure 7B, 7C). Therefore, we preliminarily conclude that both CXCL1 and CXCL8 could be taken as the CXCR2 ligand, and so participate in regulation of GC pathogenesis.
Figure 7.
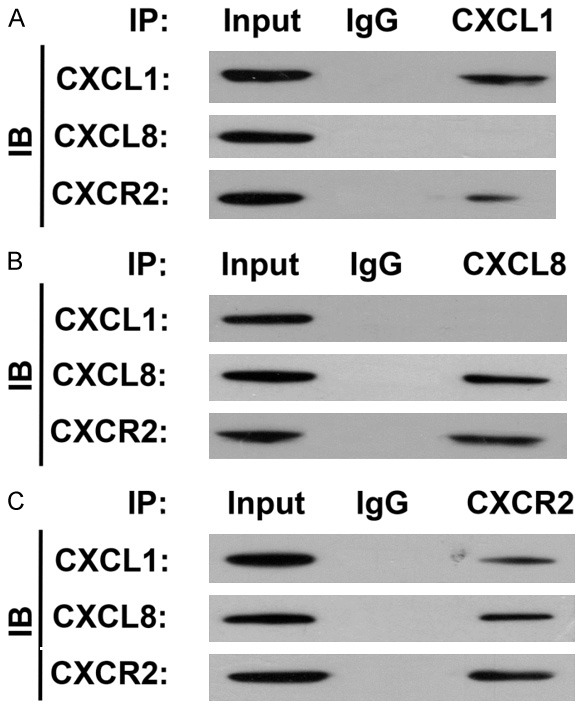
The interaction CXCL1 and CXCL8 was detected in HGC27 cells with Co-IP assay. Both CXCL1 and CXCL8 could be pulled down as the CXCR2 ligand.
CXCL1 and CXCL8 have a supplementary effect in function
In order to explore the relationship among CXCR2, CXCL1, and CXCL8 expression patterns, CXCR2 was knocked down in HGC27 cells by siRNA. W.B. results showed that the difference between CXCL1 and CXCL8 in expression change was not significant after the CXCR2 expression was inhibited by siRNA, whereas after CXCL1 expression was inhibited by siRNA, CXCL8 expression was up-regulated significantly, which indicated that after inhibition of CXCL1 expression, the cells could compensate for the functional defect through regulation of CXCL8 expression (Figure 8).
Figure 8.
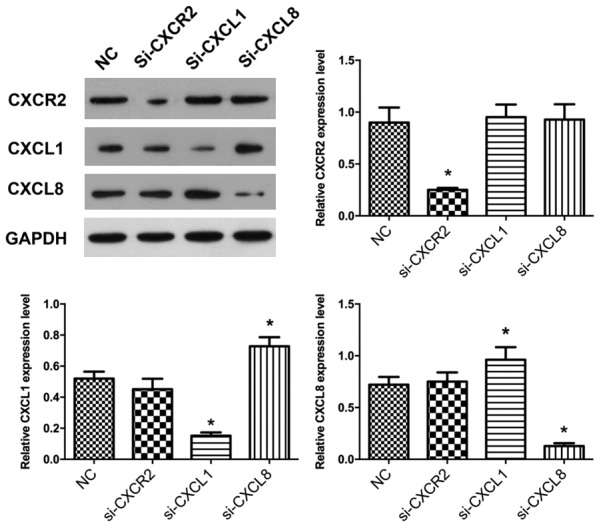
The expression of CXCL1 and CXCL8 was detected in HGC27 cells with Western Blot. Down regulation of CXCL1 or CXCL8 can induce each other’s up regulation. Compared with NC, *P < 0.05. Experiments were performed in triplicate. Data represent the mean ± S.D. from three independent experiments.
Discussion
Presently, exploration of therapeutic targets for tumors is a hot topic for cancer research. GC is a malignant tumor with a high death rate and poor prognosis [15]. The process for emergence and development of GC is very complicated. An analysis on daily routine and dietary structure showed that irregular routines and dietary habits, as well as unhealthy foods, are important external factors inducing GC [16]; an analysis on physiological and molecular mechanisms found that the internal environment and imbalance of self-monitoring mechanisms for cells were important internal factors inducing GC [17]. Although strategies for prevention of GC are becoming more and more mature clinically, it is still difficult to achieve an early diagnosis of GC accurately. Therefore, developing a new biomarker for early diagnosis of GC becomes very important.
Chemokines play a very important role in the occurrence and development of several tumors [18]. The fundamental function of chemokines is directional chemotaxis for cells expressing the chemokine receptor [19]. At present, it is believed that there are 4 steps for immunocytes to overcome the barrier of vascular endothelial cells and go through the body fluid and tissues. The cells flow along with the fluid, adhere to the vascular endothelium firmly, go through the spaces of the endothelial cells, and migrate to specific tissues [20]. In this process, chemokines control the selectivity of exudate cells and the firm adhesion of selected cells [21]. The firmly adhered immunocytes may, under action of special enzymes, go through spaces of endothelial cells and basilar membranes, and under the guidance of chemokine concentration gradients, migrate to specific tissues and change physiological features of the tissues [22,23]. It was reported that the chemokine family proteins CXCL1 and CXCL8 participate in pathogenic processes of several cancers [24,25]. In exploration of non-small cell lung cancer, the researchers verified promotion of CXCL8 on angiogenesis of tumor tissues [26]. In fact, in our pre-test studies, we found that miR-873 modulates CXCL1 expression in GC cells and this significant down-regulation changed the physiological characteristics of the cells. This indicates that CXCL1 might have an important role in the GC pathogenesis. The identified CXCL1 receptor is CXCR2. However, besides CXCL1, there is another CXCR2 ligand, namely CXCL8 [27]. In the pathogenic process of GC, the relation between CXCL1 and CXCL8 is still unclear.
In this experiment, we collected data and tissue specimens of GC patients and performed a statistical analysis, which showed that GC patients’ prognosis and survival rate were significantly correlated with CXCL1 and CXCL8 expression quantity in the specimens. Also, through the experiment, we found that for GC, as compared with in para-carcinoma tissues, the CXCL1 and CXCL8 expression quantity in GC tissues was significantly up-regulated, which indicated that both CXCL1 and CXCL8 might be proto-oncogene, but the relation between CXCL1 and CXCL8 in GC is still unclear. So, we performed CXCL1 and CXCL8 knockout in HGC27 cells through siRNA, and tested the features for cell proliferation, apoptosis, and migration again. The results showed that CXCL1 and CXCL8 had a synergistic effect in regulation of HGC27 cells proliferation, apoptosis, and migration. Both CXCL1 and CXCL8 may be taken as the CXCR2 ligand, and inhibition of the expression for one of them could induce high expression for the other. In fact, for the relation of different members of the same gene family, some have no connection with each other, some have a competitive relationship, and others have a complementary relationship in function. In the pathogenic process of GC, the effects of CXCL1 and CXCL8 were similar. Both may induce formation of tumor vessels, advance proliferation of tumor cells, and inhibit apoptosis of tumor cells. Based on above relations, as one of CXCL1 and CXCL8 expressions was inhibited, excessive receptors can recruit more other ligands for combination, which may induce high expression of another. However, in the pathogenic process of GC, it is very possible that the relationship between CXCL1, CXCL8, and CXCR2 is not the only factor for regulation of GC occurrence and development. In future studies, we will analyze the expression profile of chemokine family in GC specimens and deeply explore the influence of chemokine family on pathogenesis of GC.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81570495).
Disclosure of conflict of interest
None.
References
- 1.Abozeid M, Rosato A, Sommaggio R. Immunotherapeutic strategies for gastric carcinoma: a review of preclinical and clinical recent development. Biomed Res Int. 2017;2017:5791262. doi: 10.1155/2017/5791262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlomagno N, Incollingo P, Tammaro V, Peluso G, Rupealta N, Chiacchio G, Sandoval Sotelo ML, Minieri G, Pisani A, Riccio E, Sabbatini M, Bracale UM, Calogero A, Dodaro CA, Santangelo M. Diagnostic, predictive, prognostic, and therapeutic molecular biomarkers in third millennium: a breakthrough in gastric cancer. Biomed Res Int. 2017;2017:7869802. doi: 10.1155/2017/7869802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang YL, Yong WP, Tan P. Translating gastric cancer genomics into targeted therapies. Crit Rev Oncol Hematol. 2016;100:141–6. doi: 10.1016/j.critrevonc.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–14. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caronni N, Savino B, Recordati C, Villa A, Locati M, Bonecchi R. Cancer and chemokines. Methods Mol Biol. 2016;1393:87–96. doi: 10.1007/978-1-4939-3338-9_8. [DOI] [PubMed] [Google Scholar]
- 6.Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol. 2015;93:372–83. doi: 10.1038/icb.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, Troncoso P, Davis J, Pettaway C, Ward J, Frazier ML, Logothetis C, Kolonin MG. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourcier C, Griseri P, Grépin R, Bertolotto C, Mazure N, Pagès G. Constitutive ERK activity induces downregulation of tristetraprolin, a major protein controlling interleukin8/CXCL8 mRNA stability in melanoma cells. Am J Physiol Cell Physiol. 2011;301:C609–18. doi: 10.1152/ajpcell.00506.2010. [DOI] [PubMed] [Google Scholar]
- 9.Spaks A, Jaunalksne I, Spaka I, Chudasama D, Pirtnieks A, Krievins D. Diagnostic value of circulating CXC chemokines in non-small cell lung cancer. Anticancer Res. 2015;35:6979–83. [PubMed] [Google Scholar]
- 10.Spaks A. Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. 2017;(Suppl 3):S164–S171. doi: 10.21037/jtd.2017.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, Cassatella MA. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004;172:5034–40. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- 12.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117–29. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Jovanović M, Stefanoska I, Radojcić L, Vićovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139:789–98. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- 14.Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O’Sullivan JM, Wilson RH, Johnston PG, Waugh DJ. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther. 2008;327:746–59. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- 15.Shitara K, Ohtsu A. Advances in systemic therapy for metastatic or advanced gastric cancer. J Natl Compr Canc Netw. 2016;14:1313–1320. doi: 10.6004/jnccn.2016.0138. [DOI] [PubMed] [Google Scholar]
- 16.Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol. 2012;19:2128–34. doi: 10.1245/s10434-012-2225-6. [DOI] [PubMed] [Google Scholar]
- 17.Chang CY, Lee LJ, Wang JD, Lee CT, Tai CM, Tang TQ, Lin JT. Health-related quality of life in patients with Barrett’s esophagus. Health Qual Life Outcomes. 2016;14:158. doi: 10.1186/s12955-016-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caronni N, Savino B, Recordati C, Villa A, Locati M, Bonecchi R. Cancer and chemokines. Methods Mol Biol. 2016;1393:87–96. doi: 10.1007/978-1-4939-3338-9_8. [DOI] [PubMed] [Google Scholar]
- 19.Veldkamp CT, Koplinski CA, Jensen DR, Peterson FC, Smits KM, Smith BL, Johnson SK, Lettieri C, Buchholz WG, Solheim JC, Volkman BF. Production of recombinant chemokines and validation of refolding. Methods Enzymol. 2016;570:539–65. doi: 10.1016/bs.mie.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreau N, Mauborgne A, Bourgoin S, Couraud PO, Romero IA, Weksler BB, Villanueva L, Pohl M, Boucher Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain. 2016;157:827–39. doi: 10.1097/j.pain.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 21.Karin N, Wildbaum G. The role of chemokines in shaping the balance between CD4(+) T cell subsets and its therapeutic implications in autoimmune and cancer diseases. Front Immunol. 2015;6:609. doi: 10.3389/fimmu.2015.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amoueian S, Attaranzadeh A, Montazer M. Intratumoral CD68-, CD117-, CD56-, and CD1a-positive immune cells and the survival of Iranian patients with non-metastatic intestinal-type gastric carcinoma. Pathol Res Pract. 2015;211:326–31. doi: 10.1016/j.prp.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Peng CW, Wang LW, Fang M, Yang GF, Li Y, Pang DW. Combined features based on MT1-MMP expression, CD11b + immunocytes density and LNR predict clinical outcomes of gastric cancer. J Transl Med. 2013;11:153. doi: 10.1186/1479-5876-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, Troncoso P, Davis J, Pettaway C, Ward J, Frazier ML, Logothetis C, Kolonin MG. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz de Porras V, Bystrup S, Martínez-Cardús A, Pluvinet R, Sumoy L, Howells L, James MI, Iwuji C, Manzano JL, Layos L, Bugés C, Abad A, Martínez-Balibrea E. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep. 2016;6:24675. doi: 10.1038/srep24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Pu Y, Wang M, Hong Y, Wu Y, Tang Z. Adiponectin promotes human jaw bone marrow mesenchymal stem cell chemotaxis via CXCL1 and CXCL8. J Cell Mol Med. 2017;21:1411–1419. doi: 10.1111/jcmm.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]


