Abstract
Sinonasal teratocarcinosarcoma (SNTCS) is a rare malignancy characterized by potent invasiveness, rapid growth, and a poor prognosis. Due to its diverse histopathological morphology, complex components, and insidious growth sites, as well as inadequate biopsies, SNTCS is often misdiagnosed as other tumors, and this misdiagnosis directly affects the treatment and prognosis of patients. Here, we report a case of an elderly SNTCS patient who underwent intranasal endoscopic tumor resection under systemic anesthesia. His preoperative diagnosis and intraoperative frozen sections both led to a misdiagnosis of olfactory neuroblastoma (ONB). However, after surgeries, the patient was diagnosed with SNTCS based on routine histopathology and immunohistochemical staining. He then underwent follow-up but died of tumor recurrence six months later.
Keywords: Teratocarcinosarcoma, olfactory neuroblastoma, paranasal sinus, pathology
Introduction
Sinonasal teratocarcinosarcoma (SNTCS) is a very rare and aggressive malignant neoplasm; histologically, it has the characteristics of both teratoma and carcinosarcoma. Classical SNTCS is histologically characterized by the combination of one or many components of benign and malignant epithelial, mesenchymal, and nerve elements [1,2]. The disease posesa diagnostic challenge due to its low incidence, non-specific imaging manifestations, and complex pathological features. Here, we report one case of SNTCS in the olfactory cleft that was clinically highly similar to olfactory neuroblastoma (ONB). The patient was eventually diagnosed with SNTCS based on histopathological and immunohistochemical staining. We reviewed the relevant literature and investigated the histological origin, clinical and pathological traits, diagnosis, treatment, and prognosis for this SNTCS case.
Case report
An 80-year-old male presented to Hangzhou First People’s Hospital complaining of left nasal congestion for six months and left nostril bleeding for two days on January 18, 2011; he displayed no headache, vision loss, diplopia, or olfactory disorder. Physical examination upon admission revealed a pale red mass in the olfactory cleft of the left nasal cavity, with a slightly uneven surface and some bleeding. Computed tomography (CT) of the nasal sinus showed a soft tissue mass involving the left nasal cavity and partial absorption of the turbinate (Figure 1). Ultrasonography of the neck revealed no apparent enlargement of the lymph nodes. Based on these findings, the patient was diagnosed with ONB in the left nasal cavity. Three days after his hospitalization, the patient underwent intranasal endoscopy-associated tumor resection under systemic anesthesia. During this surgery, the tumor, which had a propensity to bleed upon being touched, was observed to be in the upper region of the left olfactory cleft and protruding downward to block the common nasal meatus. Rapidpathological diagnosis was achieved by an excisional biopsy of the tumor, which revealed the following findings: stratified squamous epithelium was present on the surface of the tumor; small nested cells had proliferated inside the mesenchyma, some of which manifested gland-like or ridge-like organization (Figure 2). Correspondingly, the possibility of ONB was considered, which warranted immunohistochemical staining for further clarification. Intraoperatively, the left middle turbinate was partially removed before the tumor was completely resected along its edge, after which the left ethmoid sinus and sphenoid sinus were opened. Smooth mucosa was present inside these sinuses, with no tumor involvement. The tumor section was gray in color and solid in texture. Microscopic examination of the surgical specimen showed the following findings: non-matured squamous cell nests were present underneath the pseudostratified ciliated columnar epithelium, and chondrocytes and adenocarcinomal structures were present in some areas; inside the tumor, there were zones of massive and dense proliferation of primary small cells with apparent cellular heteromorphology. Immunohistochemical staining revealed epithelial cells positive for CK and EMA, some of which were immunoreactive to Syn, CD99, and S-100. Positive Vim staining was found in the interstitial and small cellular areas. Positive CgA was found in the microfocal epithelial area. Staining for CD56 and NSE was positive in the small cells, some of which were immunoreactive to Syn, CD99, and S-100. The Ki-67 proliferation rate was approximately 85% (Figure 3). The patient was diagnosed with SNTCS based on postoperative pathological diagnosis and was recommended to undergo radiochemotherapy, but he refused any further treatment.
Figure 1.
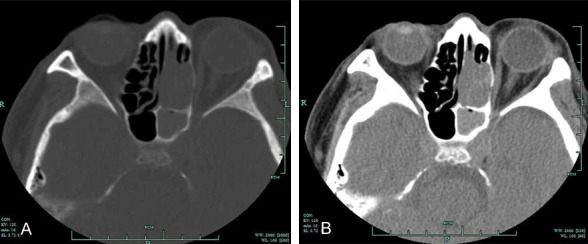
Axial CT scan of the nasal sinus revealed the following signs: a soft tissue mass filling the left nasal cavity and partial absorption of the turbinate (A: Bone window; B: Soft tissue window).
Figure 2.
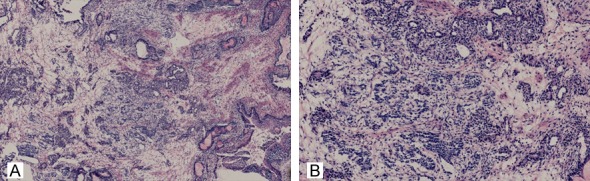
Rapid pathological examination of intraoperative frozen section showed the following findings: stratified squamous epithelium was present on the surface of the tumor; inside the mesenchyma, nested small cells had proliferated, some of which manifested gland-like or ridge-like organization. (hematoxylin and eosin [HE] staining, A: ×40; B: ×100).
Figure 3.
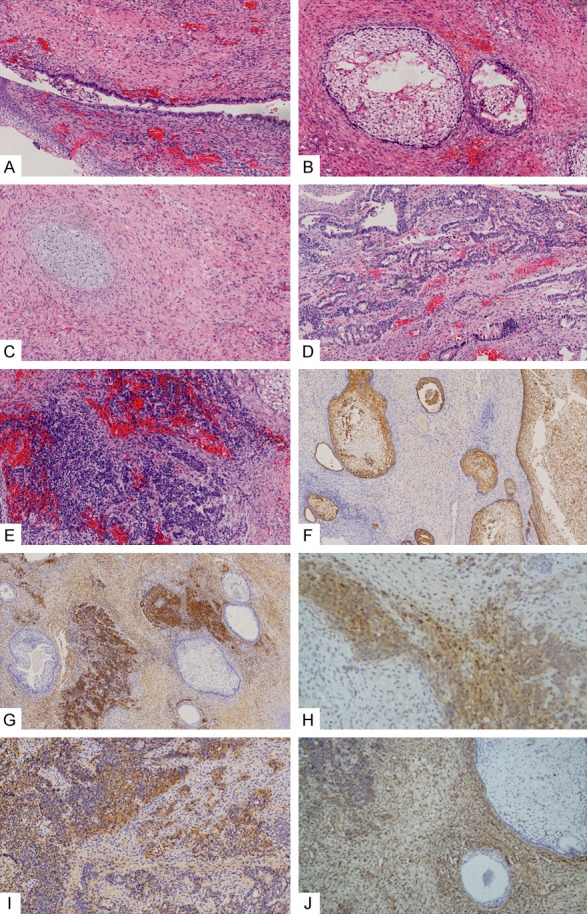
Histopathological examination revealed the following signs: (A) pseudostratified ciliated columnar epithelium on the top of (B) immature squamous cell nests, chondrocytes (C) and adenocarcinoma structure (D) visible in some subepithelial regions, and massive dense proliferation of primitive small cells (E). (A-E: HE staining, ×200). Immunohistochemical staining revealed the following results: epithelial cells positive for epithelial CK (F), small cellular regions positive for CD56 (G) and NSE (H), some epithelial cells and small cells were positive for CD99 (I), and mesenchyma and small cells positive for Vim (J) (F and G: ×100; H-J: ×200).
Five months after surgery, the patient was again hospitalized due to bleeding in both nostrils. Physical examination revealed a mass along the edge of the left posterior nostril that had a slightly uneven surface and a propensity for bleeding. The patient received a tumor biopsy in the left nasal cavity under local anesthesia. Remarkably, the results of histopathology and immunohistochemical staining were similar to those findings from the first postoperative pathological examination, which indicated recurrent SNTCS. One month after biopsy, the patient underwent enhanced magnetic resonance imaging (MRI) of the nasal sinus, which generated the following signs: i) a soft tissue manifesting iso-T1 and long-T2 signals in the bilateral nasal cavities and ii) apparently enhanced lesions, which protruded into the right orbit and had a blurred boundary with the right rectus muscle. The tumor had invaded upward into the anterior skull base and the left frontal lobe, which displayed inhomogeneous contrast enhancement. Flaky edema was present in the brain tissues around the tumor (Figure 4). The patient decided to withdraw his treatment and died 1 month later.
Figure 4.
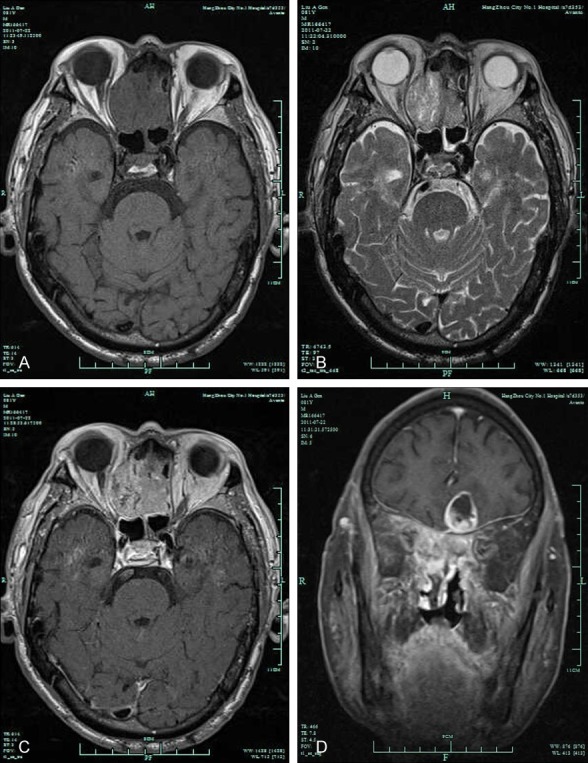
Contrast-enhanced MRI of the nasal sinus revealed the following signs: soft tissue manifesting iso-T1 and long-T2 signals in bilateral nasal cavities and an apparent enhanced lesion protruding into the right orbit and having a blurred boundary with the right rectus eye muscle. The tumor invaded upward into the anterior skull base and left frontal lobe. (A: T1-weighted MRI; B: T2-weighted MRI; and C, D: Gd-enhanced T1-weighted MRI).
Discussion
SNTCS is a very rare and aggressive malignant neoplasm that has been historically referred to as “blastoma”, “malignant teratoma”, and “teratoma”. The term “teratocarcinosarcoma” was first proposed by Heffner and Hyams in 1984. Histologically, SNTCS is characterized by the combination of one or many components of epithelial and mesenchymal elements (both benign and malignant) and also incorporates some characteristics of malignant teratoma and carcinosarcoma [3]. SNTCS was first included as a distinct entity in the World Health Organization (WHO) 2005 edition of Pathology and Genetics of Head and Neck tumors [4]. However, the tissue origin of SNTCS remains unknown. SNTCS has been proposed to originate from pluripotent stem cells that are accidentally trapped in the respiratory tract during development or as primitive embryonic tissue. A genetics study of SNTCS suggested that the occurrence of SNTCS could be related to the absence of a demonstrable amplification of chromosome 12p [5].
SNTCS is primarily found in male adults over 35 years of age and has a mean onset age of 55 years old with a male/female ratio of 7:1 [4,6]. Although SNTCS mainly occurs in the ethmoid sinus, the olfactory cleft, and the maxillary sinus, some rare cases in the nasopharynx, the pharyngeal wall, and the anterior skull base have been reported [4]. In general, SNTCS has a course of disease ranging from several weeks to 2 years. Its clinical manifestations mainly include nasal congestion, nosebleeds, and diminished olfactory function. When SNTCS invades neighboring areas such as the skull base, the cavernous sinus, and the central nervous system, other symptoms may occur, including headache, eye pain, reduced vision, and alterations in personality and behavior. CT and MRI images of SNTCS often lack any specificity. In addition, there are no specific diagnostic criteria for SNTCS in laboratory tests. In this report, the tumor location, clinical manifestations, and imaging results were remarkably similar to those of ONB, which was an important cause of the initial misdiagnosis.
Pathologically, SNTCS is often misdiagnosed as ONB, especially in small biopsies. The pathological features of ONB are relatively uniform tumor components and the presence of neuroblasts characterized by relatively primitive cell differentiation and rosette- and epithelium-like structures. Immunohistochemical staining revealed that the tumor cells were immunoreactive to S-100, NSE, Syn, CgA, whereas staining for CD99 was negative [7]. Unlike ONB, SNTCS consists of three germ layers and features distinctive histopathological traits and immunohistochemical staining. The primary feature of SNTCS is teratoma elements, which contain the following layers: i) ectoderm composed of keratinized or non-keratinized squamous epithelial neuroendocrine components, which are arranged in a duct-like architecture, with interior columnar cells; ii) mesoderm containing immature bone tissues, cartilage, smooth muscle fibers, and fibrous vascular tissues; and iii) endoderm composed of epithelium and glands from the gastrointestinal and respiratory tracts. SNTCS also has carcinosarcoma elements. As a consequence, there are some zones of adenocarcinoma and spindle cell sarcoma in addition to the teratoma. SNTCS also contains some differentiated neuroepithelial cells, which form ONB regions and neurofibrillary matrices. As such, SNTCS features myriad complex elements in various ratios and degrees of maturation and may even harbor ONB zones. Hence, the pathological diagnosis of SNTCS often requires multiple biopsies or radically resected specimens. In this study, the patient was initially diagnosed with ONB based on intraoperative frozen section findings, possibly due to inadequate specimen collection. Immunohistochemical staining of this tumor revealed CK- and EMA-labeled epithelial components, Vim-labeled smooth muscle and spindle cell sarcoma tissue, S-100-labeled neuroepithelial tissue, neuroglia and small amounts of cartilage tissue, NSE- and CgA-labeled neuroepithelial tissue and neurofibrillary matrices, and CD99-labeled small cells with low degrees of differentiation [8-10]. Therefore, the results of routine pathological and immunohistochemical staining were consistent with the pathological features of SNTCS.
Currently, there is a lack of consensus on therapeutics for SNTCS, but a combination of surgery and radiotherapy appear to be the most effective treatment option. If metastasis or recurrence occurs, chemotherapy is recommended [4,6,11-13]. Wassef et al. reported that combined surgery-radiotherapy therapeutics for SNTCS patients generated a low recurrence rate of 37% [13]. Smith et al. treated 5 SNTCS patients with a combined surgery-radiotherapy procedure and found no recurrence or distal metastasis during the 72-372 month follow-up period [11,14]. In this case, although the patient underwent radical tumor resection by transnasal endoscopic surgery, he had a survival period of only 6 months after refusing postoperative radiotherapy.
SNTCS is a highly malignant tumor that is characterized by remarkably fast growth and potent invasiveness. SNTCS often destroys the neighboring soft tissues and bone materials and may even invade the orbital cavity and intracranial region. Because it has a high likelihood of local lymph node metastasis and distal metastasis, SNTCS patients have poor prognoses. A retrospective analysis by Heffner et al. was performed in 20 cases of SNTCS with a median survival period of 1.7 years [15]. A meta-analysis of 54 SNTCS cases was performed by Wei et al., which reported that 50% of the cohort survived less than 3 years [16].
SNTCS is a very rare and special malignancy that lacks specific clinical and imaging features. Hence, it is easily misdiagnosed, which causes treatment delay. Clinicians therefore must have an increased awareness of SNTCS. If a patient exhibits signs such as unilateral nasal obstruction, headache and epistaxis with unknown causes, the possibility of malignant tumors should be immediately considered. CT and MRI of the nasal sinus and biopsy should be performed as soon as possible. SNTCS consists of a variety of complex components, and it is very important to perform repeated biopsies and to thoroughly examine surgical specimens to establish a reliable pathological diagnosis. As SNTCS has a poor prognosis, comprehensive therapeutics should be used, and close follow-up monitoring should be provided, thereby improving patients’ survival.
Acknowledgements
This work was supported by grants from Traditional Chinese Medicine Administration of Zhejiang (No. 2017ZA098), Medical and Health Technology Program of Hangzhou (No. 2014A08) and Zhejiang Provincial Natural Science Foundation of China (No. LY17H130001).
Disclosure of conflict of interest
None.
References
- 1.Chakraborty S, Chowdhury AR, Bandyopadhyay G. Sinonasal teratocarcinosarcoma: case report of an unusual neoplasm. J Oral Maxillofac Pathol. 2016;20:147–150. doi: 10.4103/0973-029X.180979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao KK, Eng TY, Barnes J, Dahiya R. Sinonasal teratocarcinosarcoma. Am J Clin Oncol. 2004;27:29–32. doi: 10.1097/01.coc.0000045851.76922.2e. [DOI] [PubMed] [Google Scholar]
- 3.Fatima SS, Minhas K, Din NU, Fatima S, Ahmed A, Ahmad Z. Sinonasal teratocarcinosarcoma: a clinicopathologic and immunohistochemical study of 6 cases. Ann Diagn Pathol. 2013;17:313–318. doi: 10.1016/j.anndiagpath.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Joshi A, Noronha V, Sharma M, Dhumal S, Juvekar S, Patil VM, Pai P, Prabhash K. Neoadjuvant chemotherapy in advanced sinonasal teratocarcinosarcoma with intracranial extension: report of two cases with literature review. J Cancer Res Ther. 2015;11:1003–1005. doi: 10.4103/0973-1482.165878. [DOI] [PubMed] [Google Scholar]
- 5.Kane SV, Karpate AA, Bal M, Juvekar SL, Pai PS. Chemotherapy-induced neuronal maturation in sinonasal teratocarcinosarcoma--a unique observation. Head Neck Pathol. 2009;3:31–36. doi: 10.1007/s12105-008-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra P, Husain Q, Svider PF, Sanghvi S, Liu JK, Eloy JA. Management of sinonasal teratocarcinosarcoma: a systematic review. Am J Otolaryngol. 2014;35:5–11. doi: 10.1016/j.amjoto.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Pai SA, Naresh KN, Masih K, Ramarao C, Borges AM. Teratocarcinosarcoma of the paranasal sinuses: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:718–722. doi: 10.1016/s0046-8177(98)90281-7. [DOI] [PubMed] [Google Scholar]
- 8.Peng G, Ke Y, Wang T, Feng Y, Li Y, Wu G. Intensity-modulated radiotherapy for sinonasal teratocarcinosarcoma. J Huazhong Univ Sci Technolog Med Sci. 2011;31:857–860. doi: 10.1007/s11596-011-0691-x. [DOI] [PubMed] [Google Scholar]
- 9.Salem F, Rosenblum MK, Jhanwar SC, Kancherla P, Ghossein RA, Carlson DL. Teratocarcinosarcoma of the nasal cavity and paranasal sinuses: report of 3 cases with assessment for chromosome 12p status. Hum Pathol. 2008;39:605–609. doi: 10.1016/j.humpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Shorter C, Nourbakhsh A, Dean M, Thomas-Ogunniyi J, Lian TS, Guthikonda B. Intracerebral metastasis of a sinonasal teratocarcinosarcoma: a case report. Skull Base. 2010;20:393–396. doi: 10.1055/s-0030-1254404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SL, Hessel AC, Luna MA, Malpica A, Rosenthal DI, El-Naggar AK. Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg. 2008;134:592–595. doi: 10.1001/archotol.134.6.592. [DOI] [PubMed] [Google Scholar]
- 12.Tokunaga T, Sunaga H, Kimura Y, Tsuda G, Fujieda S. A case of sinonasal teratocarcinosarcoma treated with surgery and post-operative intensity-modulated radiotherapy (IMRT) Auris Nasus Larynx. 2012;39:641–645. doi: 10.1016/j.anl.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Wassef SN, Kapur P, Barnett SL, Myers LL. Sinonasal teratocarcinosarcoma with intracranial extension: case report and literature review. Ear Nose Throat J. 2012;91:536–539. doi: 10.1177/014556131209101210. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, Sun R, Liang J, Zhou Z, Zhou J, Rui J. Sinonasal teratocarcinosarcoma: a clinical and pathological analysis. Int J Surg Pathol. 2013;21:37–43. doi: 10.1177/1066896912457202. [DOI] [PubMed] [Google Scholar]
- 15.Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses A clinicopathologic study of 20 cases. Cancer. 1984;53:2140–2154. doi: 10.1002/1097-0142(19840515)53:10<2140::aid-cncr2820531025>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Wei S, Carroll W, Lazenby A, Bell W, Lopez R, Said-Al-Naief N. Sinonasal teratocarcinosarcoma: report of a case with review of literature and treatment outcome. Ann Diagn Pathol. 2008;12:415–425. doi: 10.1016/j.anndiagpath.2007.05.003. [DOI] [PubMed] [Google Scholar]


