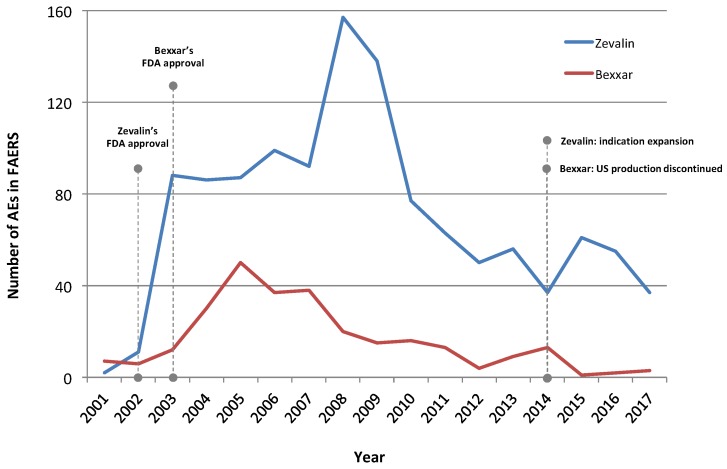
Figure 1.
Timeline of AEs reporting Zevalin and Bexxar in the FDA’s Adverse Event Reporting System (FAERS). Overall, there are more AEs identified for Zevalin in FAERS, likely indicating broader usage adoption than for Bexxar. Fourteen cases reporting Bexxar prior to 2001 are not displayed. Also, the distribution of AEs over time does not include incompletely reported cases for which no date was specifically registered. Last, AEs dated prior to a drug’s approval may reflect reports from preapproval studies and clinical trials. The reduction of AE numbers for 2017 is explained by the fact that the full dataset for that year was not yet released by FAERS at the time this analysis took place. Note that a certain time delay between the disclosure of reports to FAERS and the date of an event or the use of an agent is reasonable to be expected.