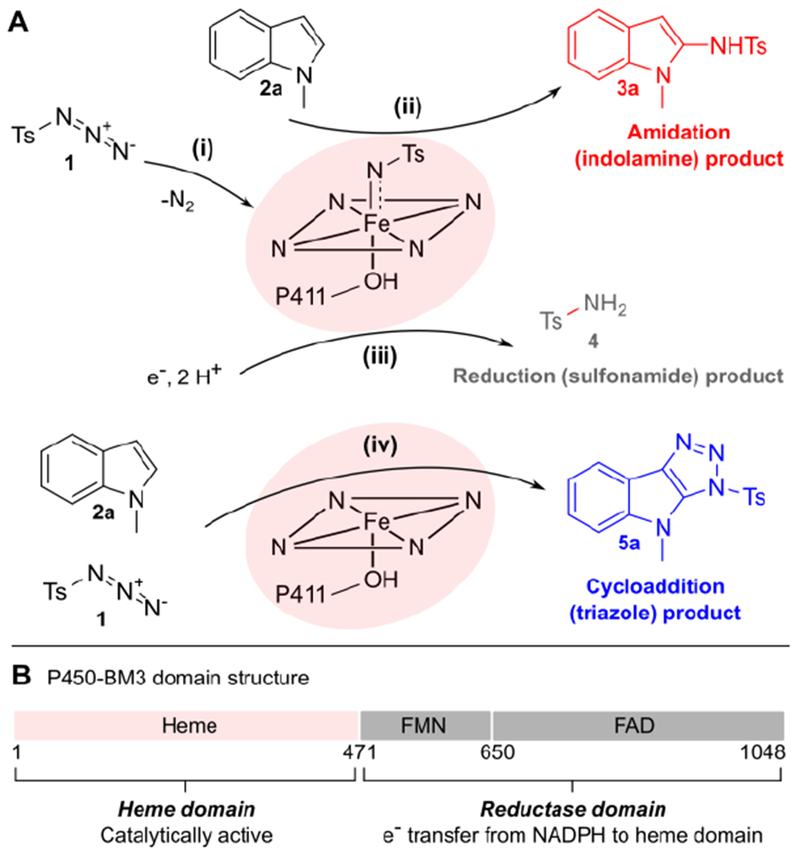
Figure 1: Engineering chemoselectivity in P411-catalyzed indole amidation.
(A) Proposed reaction scenarios: tosyl azide 1 can react with the P411 iron heme cofactor to form an iron-nitrenoid intermediate (i). The nitrene can react with 1-methylindole 2a to yield the desired amidation product 3a (ii) or it can be reduced to give sulfonamide product 4 (iii). Alternatively, 1 and 2a can react to give cycloaddition product 5a (iv); while this reaction is likely accelerated by the P411 active site (vide infra), the involvement of the heme cofactor remains to be determined. (B) P450-BM3 domain structure; we targeted both the heme and reductase domains for directed evolution of chemoselective formation of 3a.