Abstract
Autoimmune hemolytic anemia (AIHA) is a rare disease in which autoantibodies target red blood cells (RBCs), leading to anemia that ranges from no symptoms to severe life-threatening hemolysis. Little is known about the severity of anemia, blood transfusion efficiency and risk of transfusion-related reactions among hospitalized AIHA patients, especially in those with incompatible RBC transfusions.
A retrospective study was conducted among hospitalized AIHA patients from January 2009 to December 2015 in a large tertiary care medical center in southwest China.
A total of 450 AIHA hospitalized patients were recruited, of whom 97.3% had warm AIHA, 30.3% had primary AIHA, and 90.7% were treated with corticosteroids. On admission, approximately 3% of patients had an hemoglobin (Hb) <30 g/L, 34% had an Hb between 30 and 59.9 g/L, and 46% had an Hb ranging from 60 to 89.9 g/L. A total of 2509.5 U RBCs were transfused to AIHA patients, and 14 transfusion-related adverse reactions were recorded, without any hemolytic transfusion reactions. With an average transfusion trigger of 52.0 ± 9.3 g/L, 59.7% of the patients received RBCs, and 55.8% of the transfusions were viewed as effective. Least incompatible RBCs were given in 39% of the transfusions, but the transfusion efficiency did not significantly decrease with these incompatible blood transfusions (P = .253). Primary AIHA patients with a nadir Hb of approximately 40 to 50 g/L during their hospital stay had the highest rate of remission and did not require a different total number of RBC transfusions (P = .068) or length of hospitalization (P = .194) compared to other groups with nadir Hb values <30 g/L, ≥30 and <40 g/L, ≥50 and <60 g/L, and ≥60 g/L.
One-third of AIHA patients suffered from severe anemia during hospitalization, and transfusions, even with incompatible RBCs, were safe and efficient. However, transfusion triggers between 40 and 50 g/L seemed to benefit the most patients by alleviating the RBC destruction caused by autoantibodies, and a restrictive transfusion strategy was beneficial in AIHA patients.
Keywords: autoimmune hemolytic anemia, RBC transfusion
1. Introduction
Autoimmune hemolytic anemia (AIHA) is a rare autoimmune disease characterized by an increased destruction of red blood cells (RBCs) mediated by autoantibodies against autologous RBCs. The incidence of AIHA in adults is 1 to 3 cases per 100,000 per year.[1] AIHA can be classified according to serologic or clinical characteristics. Approximately 75% to 80% of AIHAs are caused by warm autoantibodies, and the others result from cold or mixed autoantibodies.[2] In addition, AIHA can be subdivided into primary and secondary AIHA depending on whether there is an underlying disease.[3] Anemia caused by AIHA ranges from mild to severe. In some cases, the anemia is gradually or fully compensated. However, there are still some patients who develop life-threatening anemia, and RBC transfusions are urgently needed.
Blood transfusions for AIHA patients are challenging. The first problem is the difficulty in providing the patients with serologically compatible RBCs since the autoantibodies react to all screening cells and donor cells. Moreover, the autoantibodies also destroy donor RBCs, which may aggravate the hemolysis. The second problem is the increased risk for hemolytic transfusion reactions (HTRs) in these patients. It was reported that alloantibodies were found in 20% to 40% of AIHA patients.[4,5] The presence of autoantibodies can mask the presence of alloantibodies. Specialized compatibility testing procedures that allow for the detection of alloantibodies in patients with autoantibodies are time-consuming, expensive, and technically challenging. Moreover, these procedures cannot be routinely performed in most transfusion departments. The transfusions for AIHA patients are always delayed, which may sometimes lead to death.[6] To date, the decision for transfusions in AIHA patients is still mainly empirical and is based on the clinician's opinion.[7,8]
Instead of performing complicated pretransfusion testing, selecting the weakest agglutinated RBC units during the cross-match is a compromising alternative procedure conducted in many laboratories.[9] However, we do not know which approach is better because there are few reports on transfusions for hospitalized AIHA patients, and the effects and adverse reactions are also unknown.
Therefore, we conducted this study with hospitalized AIHA patients by reviewing the comprehensive data, including demographic characteristics, diagnosis, treatments, and outcomes, to assess the effectiveness and adverse reactions of transfusion. The relationship between transfusion triggers and patient outcomes was also evaluated in this study.
2. Methods
2.1. Patients
A retrospective review was conducted of all hospitalized patients diagnosed with AIHA in West China Hospital from January 2009 to December 2015. The study was approved by the ethics committee of West China Hospital, Sichuan University. The patients fulfilled the following eligibility criteria:
Diagnosis of AIHA;
Hemoglobin (Hb) level ≤110 g/L with features of hemolysis (elevated lactate dehydrogenase [LDH] level and/or elevated total bilirubin [TBIL] and indirect bilirubin level) and a positive direct antiglobulin test (DAT) result with an IgG/C3d pattern; and
Absence of any other causes of acquired or hereditary hemolytic anemia, such as paroxysmal nocturnal hemoglobinuria and glucose 6-phosphate dehydrogenase deficiency. Patients with a negative DAT result but with the other aforementioned criteria for AIHA were included if steroid treatment was effective. Data were collected from the hospital information system (HIS) and laboratory information system, including the complete clinical, laboratory and transfusion information for all patients treated at the hospital.
2.2. Secondary AIHA
Patients who met the above eligibility criteria but had an underlying disease that caused immune destruction of RBCs were classified as secondary AIHA. All diagnoses were extracted from the HIS, and the secondary AIHA patients were categorized by their main diagnosis.
2.3. Treatment
The following treatments were used:
Steroids (either via oral or intravenous routes);
Splenectomy;
Rituximab;
Immunosuppressant drugs;
Immunoglobulin;
Plasma exchange; and
Cytotoxic drugs.
Other than steroids, the other treatments were viewed as second-line therapies. In addition, transfusions were also recorded.
2.4. Outcome assessment
We assessed the treatment outcomes according to the following criteria at the end of hospitalization. A complete response (CR) was defined as an Hb level ≥120 g/L. A partial response (PR) was defined as an Hb level ≥100 g/L or an increase of at least 20 g/L from the baseline value. The clinical outcomes included death and remission. Remission was defined as CR or PR at the end of hospitalization.
2.5. Transfusion reactions
The transfusion reactions were defined and classified according to the American National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol V2.4 National Healthcare Safety Network Manual, 2017.
2.6. Transfusion effectiveness and HTR risk estimation
The transfusion was defined as effective when there was an increase in the Hb level of at least 5 g/L per unit of RBC (1 unit consisted of RBC derived from 200 mL of whole blood) transfused within 24 hours in patients without active bleeding.
A scoring system was created to estimate the risk for potential masked alloantibody-related HTR and was defined as the following: negative screening tests had a value of 0, screening tests with 1 or 2 cells reactive and at least 1 cell nonreactive had a value of 1, screening tests with all 3 cells reactive but with no patient history of transfusion or pregnancy had a value of 2, and screening tests with all 3 cells reactive and a patient history of transfusion or pregnancy had a value of 3. When a patient's HTR score reached 3, the least-incompatible blood was selected by testing the reactivity of the patient's serum against >8 ABO-compatible units and choosing those that were best matched for transfusion. Patients with an HTR score of 2 were provided with ABO-compatible units, and the others were provided with cross-matched RBC.
2.7. Statistical analysis
Pearson Chi-square tests were used to compare categorical variables. The Mann–Whitney U test and Kruskal–Wallis test were used to compare the median (range) value of continuous variables between 2 groups and among more than 2 groups, respectively. Multivariate regression analysis was applied to explore which factors were associated with the outcomes of AIHA, such as age, sex, hospital stay, transfusion, treatment, Hb level at admission, classification of AIHA (primary or secondary), and the lowest Hb during hospitalization. All statistical analyses were performed using SPSS 20 for Windows (SPSS Inc., Chicago, IL), and P values <.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of the patients
A total of 450 hospitalized patients met the eligibility criteria, with a median age of 51 years (quartile 34 –64 years) and a median hospital stay of 17 days (quartile 11–29 days). Among them, 315/450 (78.5%) were females, 438/450 (97.3%) had warm AIHA, and 321/450 (70.7%) had AIHA secondary to an underlying disorder or condition.
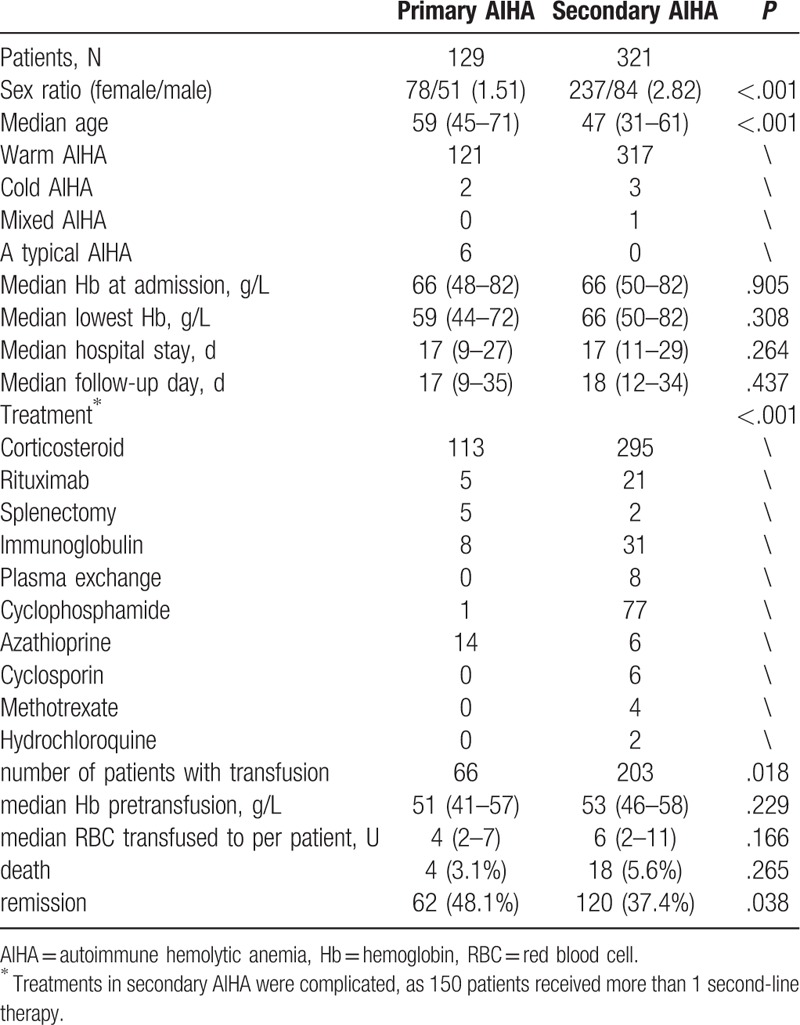
As shown in Table 1, the secondary AIHA patients were younger than the primary AIHA patients. However, compared to the primary AIHA patients, the secondary AIHA patients had a higher rate of blood transfusion, more frequent second-line therapy and poorer remission rate.
Table 1.
The baseline characteristics of primary and secondary AIHA patients.
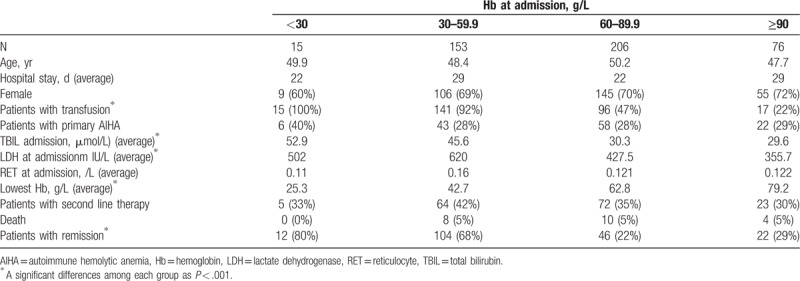
To understand the severity of their anemia, the patients were divided into 4 groups according to their Hb levels at admission: Hb < 30 g/L (15/450, 3%), Hb 30 to 59.9 g/L (153/450, 34%), Hb 60 to 89.9 g/L (206/450, 46%), and Hb ≥90 g/L (76/450, 16.9%) (Table 2). As expected, the concentration of TBIL and LDH increased with the severity of anemia (P < .001). The lowest Hb values during hospitalization decreased progressively with as the anemia worsened (P < .001), whereas the following variables showed no differences among groups: age (P = .561), sex (P = .949), type of therapy (P = .263), reticulocyte count on admission (P = .113), primary/secondary AIHA (P = .957), hospital stays (P = .771), and death (P = .992). The transfusion rate increased with the severity of anemia on admission (P < .001) and was higher than 92% in patients with Hb < 60 g/L but dropped to less than 47% in patients with Hb > 60 g/L. Moreover, the remission rate was higher in groups with Hb < 60 g/L than in those with Hb > 60 g/L.
Table 2.
Severity of AIHA patients at admission.
3.2. Cause of secondary AIHA
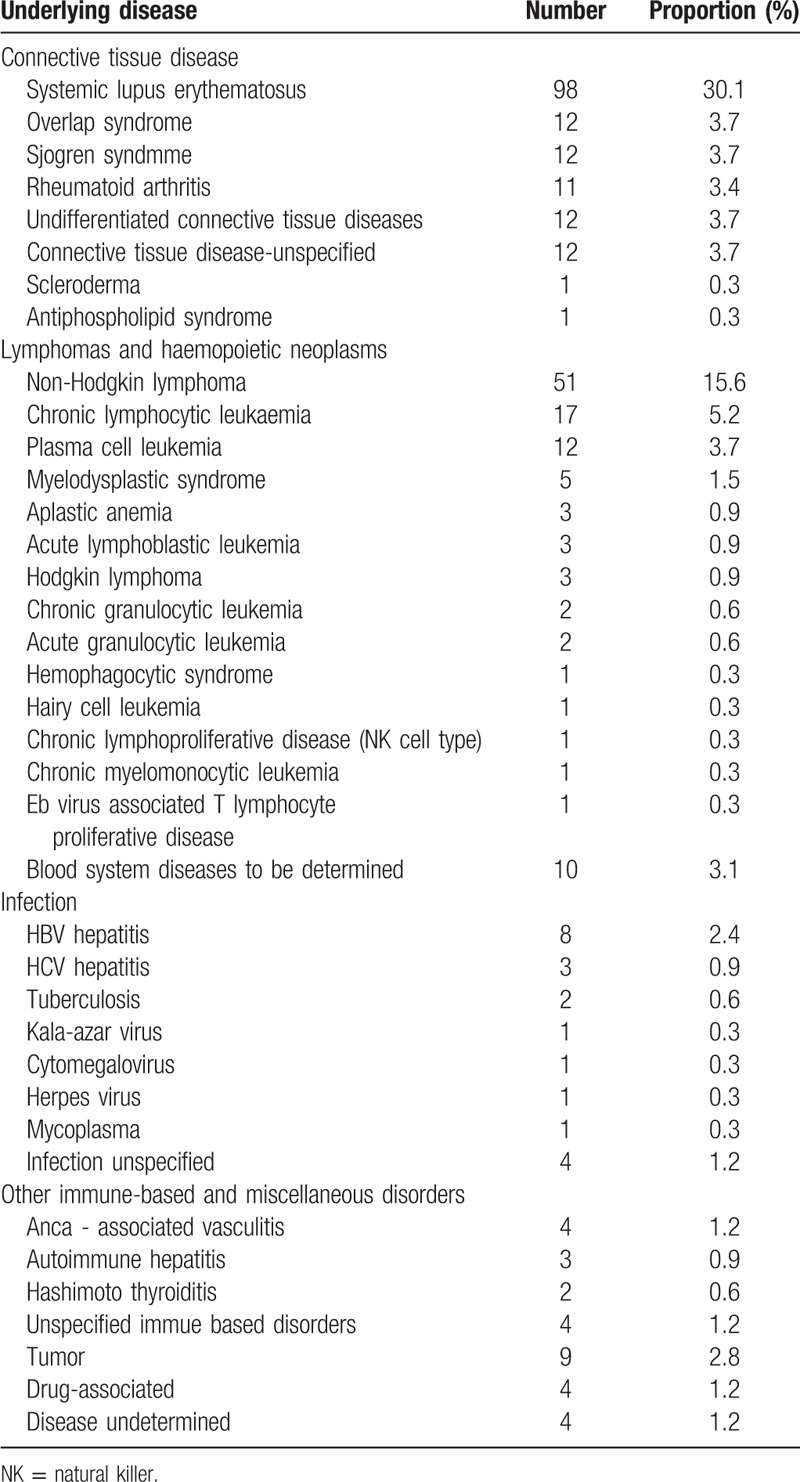
Almost half of the secondary AIHA cases were associated with connective tissue diseases 159/321 (48.8%), one-third of them were lymphomas and hemopoietic neoplasms (113/321, 34.7%), followed by infectious diseases (19/321, 5.8%), other immune-based and miscellaneous disorders (13/321, 4%), tumors (9/321, 2.8%), drug-associated disease (4/321, 1.2%), and undetermined diseases (4/321, 1.2%) (see Table 3). In general, the most common diseases seen in secondary AIHA patients were systemic lupus erythematosus (SLE) (30.1%), non-Hodgkin lymphoma (15.6%) and chronic lymphocytic leukemia (5.3%).
Table 3.
Underlying disease of secondary AIHA.
3.3. Treatment
Overall, 408/450 (90.7%) AIHA patients received corticosteroids as first-line treatment. Prednisone (1 mg/kg or 40 mg/d) or dexamethasone (10 mg/d or 15 mg/d) were the 2 most frequently used steroids. Second-line therapies were administered to 150 of 450 (33.3%) patients in combination with steroid treatment, such as steroid-sparing or steroid-substituting agents, among which rituximab was given to 26/450 (6%) patients. Splenectomy was performed in 7 patients, 4 patients had enlarged spleens, 1 suffered from active idiopathic thrombocytopenic purpura in the setting of Evans’ syndrome, 1 developed splenic infarction, and the last one showed no response with dexamethasone (15 mg/d), azathioprine or cyclophosphamide treatment. Generally, fewer primary AIHA patients were treated with second-line therapies than those with secondary AIHA (see Table 1). In addition, 27 patients did not receive corticosteroids or second-line therapy for AIHA, and 17 of them had severe infections.
3.4. Outcomes of AIHA and the associated risk factors
A total of 182 (40.4%) patients achieved remission at the end of hospitalization, with only 12 (2.7%) achieving CR. Twenty-two (4.9%) patients died, among whom 20 died of infection, 1 died of intracranial hemorrhage, and 1 died from inspiration asphyxia.
In the multivariate regression analysis, primary AIHA (P = .012, 95% confidence interval [CI]: 1.144–3.034) was associated with a favorable remission rate, while low Hb on admission (P < .001, 95% CI: 0.960–0.985) and nadir Hb during hospitalization (P < .001, 95% CI: 0.955–0.984) were associated with poor remission. In the analysis to identify risk factors for patient death, age was the only factor significantly associated with death (P = .005, 95% CI: 0.929–0.990).
3.5. Transfusion reaction assessment
A total of 2509.5 U RBCs were transfused to 269 patients in 1112 episodes. The transfusion medical records with information on temperature, Hb level, symptoms and sign changes 24 hours after the transfusion were available for 885 of the 1112 episodes. In total, 14/885 (1.6%) led to transfusion-related adverse reactions, including 13 cases of febrile reactions, 1 case of an allergic reaction, and 1 case of somatic symptoms (headache and blood pressure elevation) but no other hemolytic blood transfusion reactions. A total of 148 transfusion episodes were prophylactically supplemented with dexamethasone or promethazine. There was no significant difference in the occurrence of adverse transfusion reactions between the groups with and without prophylactic medication (2/148 vs 12/737, X2 = 0.781, P = .563).
3.6. Transfusion efficiency and HTR risk estimation
Two hundred sixty-nine out of 450 (59.7%) patients received RBC transfusions, and the Hb level increased from 52.0 ± 9.3 g/L to 65.1 ± 11.9 g/L. Each patient received a median of 5 (quartile 5–10) units RBC during the hospital stay. Transfusion efficiency was evaluated in 352 transfusion episodes, and 58.5% of them were considered efficient.
A total of 157 transfusion episodes had positive antibody screening tests, among which the 3 cells were all positive in the screening tests of 138 transfusions. The HTR risk score was distributed among the 352 transfusions as follows: 55.1% scored 0, 5.4% scored 1, 9.0% scored 2, and 30.5% scored 3. However, the transfusion efficiency did not significantly change with the HTR risk score (P = .253). This may contribute to the fact that the least incompatible crossmatching strategy effectively reduced the potential alloantibody-associated HTR risk.
3.7. Transfusion in primary AIHA patients
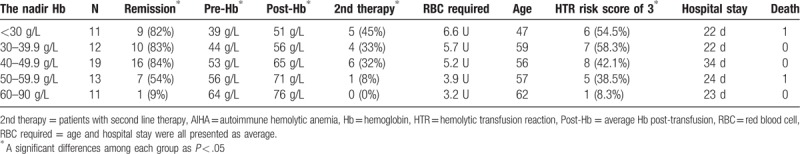
The nadir Hb of primary AIHA patients was stratified into 5 groups as per protocol: <30 g/L, ≥30 and <40 g/L, ≥40 and <50 g/L, ≥50 and <60 g/L, and ≥60 g/L (as shown in Table 4). In the group with worsening nadir Hb levels, the average Hb levels before transfusion also progressively decreased (P < .001). The remission rate (P < .001), second-line therapy rate (P = .009), and frequency of an HTR risk score of 3 (P = .004) were all higher in the group with lower nadir Hb levels than in the group with high levels, while the age (P = .446), total number of RBC transfusions (P = .068), and length of hospital stay (P = .194) were not significantly different among these groups. Patients with nadir Hb values between 40 and 50 g/L showed the best remission rate, but a further decrease in nadir Hb was not associated with an increase in remission rate (as shown in Table 4).
Table 4.
Transfusion in primary AIHA patients.
4. Discussion
The most concerning problems in the pre transfusion testing of AIHA are the presence of underlying alloantibodies, the RBC compatibility between patient sera and donor RBCs, and the potential for HTR. Limited clinical trials have focused on transfusion practices in AIHA patients. Our study provides data showing that transfusions with the least incompatible blood did not adversely affect transfusion efficiency. This is important for clinicians since there should be little delay in transfusions, should they be deemed necessary.
Selecting RBCs for transfusion according to the “least incompatible units” is controversial. This approach has been reported to be less sensitive than the adsorption method in detecting underlying clinically significant alloantibodies (particularly when the alloantibody titer is lower than that of the autoantibodies).[9] However, a previous study showed in a 7-day follow-up that transfusions with the least incompatible RBCs for AIHA patients were as effective as RBC transfusions in a control group with patients positive for alloantibodies only or those without RBC-specific antibodies.[10] Another study found that 53 patients with detectable autoantibodies did not have a definite increase in hemolysis within the first 6 months of transfusion, even when the transfused RBC were serologically incompatible.[11] In our study, the transfusion efficiency was not associated with HTR risk when least incompatible units were provided. Our finding supported that selecting the least incompatible units for transfusions was effective in preventing patients from developing HTR. Given the feasibility and cost effectiveness of this method, the practice of selecting RBCs for AIHA patients by least incompatible crossmatching is reasonable. Nevertheless, the procedure of providing phenotype/genotype-matched RBCs or performing complex procedures of serologic work-ups with adsorption can theoretically provide an increased level of safety by decreasing the potential HTRs. The mechanism of AIHA is still unknown, as only a small portion of autoantibodies cause hemolysis.[12,13] Further studies to identify the specific autoantibodies that will become clinically significant could offer a great advantage to guiding the practices for RBC-selection strategies.
Setting an Hb level as the trigger for RBC transfusions with more restricted thresholds for AIHA patients than healthy controls is supported by this study. The remission rate was higher in AIHA patients with Hb < 60 g/L at admission. We further support that an Hb value between 40 and 50 g/L was the best transfusion threshold for AIHA patients, as the best remission rate was obtained in primary AIHA patients with a nadir Hb between 40 and 50 g/L. Remission was evaluated at the end of the hospitalization period, with a median length of 17, which mostly resulted from Hb changes from transfusion. A previous study showed that 14 patients with initial Hb levels <50 g/L had more Hb changes at day 2 post transfusion than those Hb levels >50 g/L and suggested that patients with severe anemia consistently exhibited a significantly greater degree of transfusion benefits than those with mild-moderate anemia.[10] Unlike a previous study that focused on the results of 1 transfusion episode, we focused on the results of transfusions through a hospitalization period, and we used the nadir Hb to reflect the transfusion trigger.
It is highly important whether transfusions for AIHA patients are associated with an increased risk for transfusion reactions, but it has never been reported. Generally, the adverse transfusion reaction rates were comparable to those from the NHSN Hemovigilance Module in the United States, which reported 5136 adverse reactions among 2,144,723 components transfused (239.5/100,000), in which allergic and febrile nonhemolytic reactions were most frequent.[14] However, transfusion premedication was implemented in 10% of the AIHA patients, which was twice that reported in routine clinic RBC transfusion practices.[15] Consistent with a previous study that showed that transfusion premedication to prevent transfusion reactions was not supported by evidence,[16] our results showed that prophylactically using medication to reduce the rate of transfusion reactions was also unnecessary in AIHA patients.
In contrast to a previous study, our study provided a new view on the outcomes of AIHA patients. We need to note that the remission rate in our study represented the midterm outcome and that patients with remission still need therapy in the future. Remission, defined as achieving CR or PR without transfusion, steroids or second-line treatment, was estimated to be achieved in less than 70% of patients with steroids alone, as 157/450 (29.6%) patients received at least 1 second-line therapy, which was mostly applied after a failure to respond to steroids. In a study with a long follow-up, remission was observed in 75% of the patients who took steroids, whose median time to achieve PR was 15 days and that to CR was 40 days.[17]
The baseline characteristics of our population were mostly similar to those already reported, except for the relatively higher frequency of SLE in secondary AIHA patients and lower incidence of cold AIHA.[18,19] SLE was the most common disease observed for secondary AIHA and was mostly reported in Asian regions,[20,21] suggesting an important clue for diagnosis.[22–24] It is also worth noting that far fewer patients used rituximab and underwent splenectomy in our study than in a previous study.[17,25]
The main limitation of this study was that the observation period ended when patients were discharged. As a tertiary medical center, patients came from a vast region of Western China and easily discontinued their follow-up visits when they left the hospital. Although the outcome of each patient in a long-term follow-up cannot be drawn by our study, we provided a cross-sectional analysis with a large sample size to understand the AIHA presentations in hospitalized patients and their severity, classification, demographics, transfusion efficiency, transfusion adverse reactions, and remission rate. In addition, the study was also limited from its retrospective study design. For example, the transfusion efficiency analysis could be affected by missing data, as the records of Hb in the pre transfusion period or within 24-hour post transfusion were lacking. Further comprehensive prospective studies are warranted to confirm this finding.
In conclusion, we conducted the largest retrospective study on hospitalized AIHA patients with comprehensive data of the demographic characteristics, diagnosis and treatment and found that the rate of transfusion reactions did not increase and that the transfusions were effective, even in those with high HTR risk, with the use of least incompatible RBCs; moreover, those with transfusion triggers between 40 and 50 g/L seemed to suffer least from RBC destruction caused by autoantibodies.
Author contributions
Data curation: Bing Han, Li Qin, Binwu Ying.
Formal analysis: Chunxia Chen, Lixin Wang, Li Qin, Binwu Ying.
Investigation: Lixin Wang, Bing Han.
Methodology: Chunxia Chen, Lixin Wang, Bing Han, Li Qin, Binwu Ying.
Project administration: Chunxia Chen, Lixin Wang, Bing Han.
Resources: Bing Han.
Software: Bing Han.
Supervision: Li Qin.
Validation: Chunxia Chen, Lixin Wang, Li Qin, Binwu Ying.
Writing – original draft: Chunxia Chen, Lixin Wang.
Writing – review and editing: Li Qin, Binwu Ying.
Footnotes
Abbreviations: AIHA = autoimmune hemolytic anemia, CR = complete response, Hb = hemoglobin, HTR = hemolytic transfusion reaction, ITP = idiopathic thrombocytopenic purpura, LDH = lactate dehydrogenase, NHSN = national healthcare safety network, PR = partial response, RBC = red blood cell, SLE = systemic lupus erythematosus, TBIL = total bilirubin.
How to cite this article: Chen C, Wang L, Han B, Qin L, Ying B. Autoimmune hemolytic anemia in hospitalized patients: 450 patients and their red blood cell transfusions. Medicine. 2020;99:2(e18739).
CC and LW contributed equally to this article.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Hill QA, Stamps R, Massey E, et al. Guidelines on the management of drug-induced immune and secondary autoimmune, haemolytic anaemia. Br J Haematol 2017;177:208–20. [DOI] [PubMed] [Google Scholar]
- [2].Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am 2017;101:351–9. [DOI] [PubMed] [Google Scholar]
- [3].Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol 2011;4:607–18. [DOI] [PubMed] [Google Scholar]
- [4].Sokol RJ, Hewitt S, Booker DJ, et al. Patients with red cell autoantibodies: selection of blood for transfusion. Clin Lab Haematol 1988;10:257–64. [DOI] [PubMed] [Google Scholar]
- [5].Laine ML, Beattie KM. Frequency of alloantibodies accompanying autoantibodies. Transfusion 1985;25:545–6. [DOI] [PubMed] [Google Scholar]
- [6].Yurek S, Mayer B, Almahallawi M, et al. Precautions surrounding blood transfusion in autoimmune haemolytic anaemias are overestimated. Blood Transfus 2015;13:616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petz LD. A physician's guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6. [DOI] [PubMed] [Google Scholar]
- [8].Crowther M, Chan YL, Garbett IK, et al. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood 2011;118:4036–40. [DOI] [PubMed] [Google Scholar]
- [9].Ziman A, Cohn C, Carey PM, et al. Warm-reactive (immunoglobulin G) autoantibodies and laboratory testing best practices: review of the literature and survey of current practice. Transfusion 2017;57:463–77. [DOI] [PubMed] [Google Scholar]
- [10].Park SH, Choe WH, Kwon SW. Red blood cell transfusion in patients with autoantibodies: is it effective and safe without increasing hemolysis risk? Ann Lab Med 2015;35:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salama A, Berghofer H, Mueller-Eckhardt C. Red blood cell transfusion in warm-type autoimmune haemolytic anaemia. Lancet 1992;340:1515–7. [DOI] [PubMed] [Google Scholar]
- [12].Chadebech P, Loustau V, Janvier D, et al. Clinical severity in adult warm autoimmune hemolytic anemia and its relationship to antibody specificity. Haematologica 2018;103:e35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wikman A, Axdorph U, Gryfelt G, et al. Characterization of red cell autoantibodies in consecutive DAT-positive patients with relation to in vivo haemolysis. Ann Hematol 2005;84:150–8. [DOI] [PubMed] [Google Scholar]
- [14].Harvey AR, Basavaraju SV, Chung KW, et al. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion 2015;55:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fujiwara SI, Kino S, Tanaka A, et al. A national survey of premedication for transfusion reactions in Japan. Transfus Apher Sci 2017;56:708–12. [DOI] [PubMed] [Google Scholar]
- [16].Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion 2007;47:1089–96. [DOI] [PubMed] [Google Scholar]
- [17].Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood 2014;124:2930–6. [DOI] [PubMed] [Google Scholar]
- [18].Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre. Br Med J 1981;282:2023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roumier M, Loustau V, Guillaud C, et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single-center experience with 60 patients. Am J Hematol 2014;89:E150–5. [DOI] [PubMed] [Google Scholar]
- [20].Rattarittamrong E, Eiamprapai P, Tantiworawit A, et al. Clinical characteristics and long-term outcomes of warm-type autoimmune hemolytic anemia. Hematology 2016;21:368–74. [DOI] [PubMed] [Google Scholar]
- [21].Baek SW, Lee MW, Ryu HW, et al. Clinical features and outcomes of autoimmune hemolytic anemia: a retrospective analysis of 32 cases. Korean J Hematol 2011;46:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gormezano NW, Kern D, Pereira OL, et al. Autoimmune hemolytic anemia in systemic lupus erythematosus at diagnosis: differences between pediatric and adult patients. Lupus 2017;26:426–30. [DOI] [PubMed] [Google Scholar]
- [23].Skare T, Picelli L, Dos Santos TAG, et al. Direct antiglobulin (Coombs) test in systemic lupus erythematosus patients. Clin Rheumatol 2017;36:2141–4. [DOI] [PubMed] [Google Scholar]
- [24].Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alonso HC, Manuel AV, Amir CG, et al. Warm autoimmune hemolytic anemia: experience from a single referral center in Mexico City. Blood Res 2017;52:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


