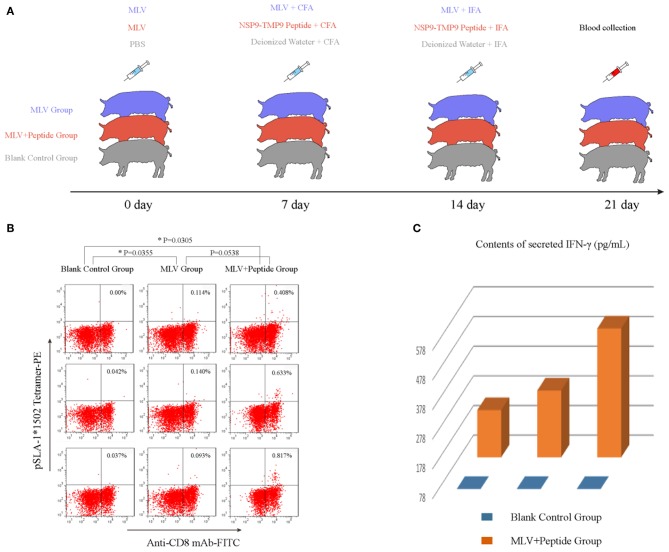
Figure 8.
Identification of the functional NSP9-TMP9 CTL epitope. (A) Immunization program for SPF pigs. Nine pigs expressing SLA-1*1502 were divided into three groups: the MLV group, MLV+Peptide group and blank control group. The MLV and MLV+Peptide groups were injected with an attenuated PRRSV vaccine as the first immunization. Seven days later, the MLV+Peptide group was injected with the NSP9-TMP9 peptide mixed with CFA, and the MLV group was injected with MLV mixed with CFA as the second immunization. At day 14, the MLV+Peptide group was injected with the NSP9-TMP9 peptide mixed with IFA, and the MLV group was injected with MLV mixed with IFA as the third immunization. Pigs in control group were injected with PBS, deionized water mixed with CFA, and deionized water mixed with IFA. (B) NSP9-TMP9-specific CTLs stained with PE-labeled SLA-1*1502 tetramer and FITC-labeled anti-CD8 monoclonal antibody were detected via flow cytometry. (C) The secreted IFN-γ contents of the control group and immunized group were measured via ELISA kit. The secreted IFN-γ content of the control group was less than the lowest detectable limit (78 pg/mL).