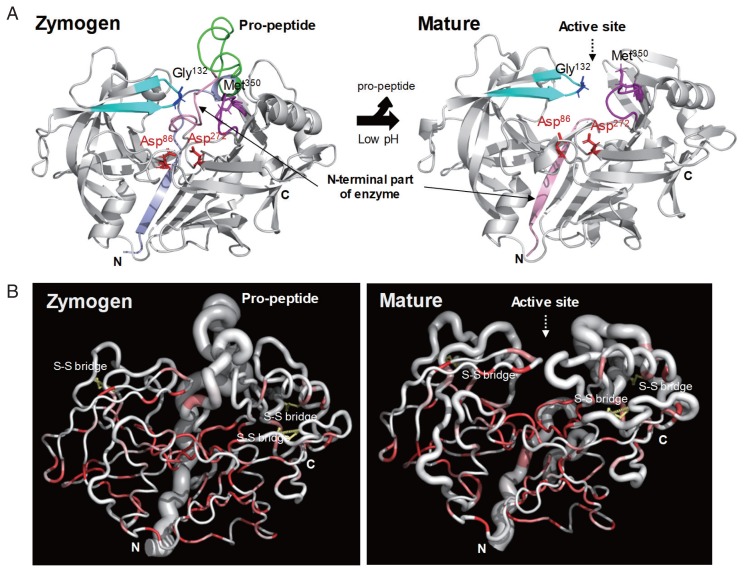
Fig. 5.
Overall structural features of CsCatD2. (A) Overall 3D structure and secondary structural features of zymogen (A) and mature (B) forms of CsCatD2. The pro-peptide region in zymogen represented as conserved part A (light navy ribbon) and variable part B (green). The Y flap region (cyan) and the polyproline loop (purple) along with the 2 catalytic aspartic residues (red stick) are also marked. The flap tip residue and hinge residue of the polyproline loop are indicated by black and purple sticks, respectively. (B) Sausage representation of the CsCatD2 with sequence and structural conservation rendering. To visualize sequence conservation within the CaCatD2 and CatD family proteins (PDB entries 5UX4, 5N7N, 5N7Q, 5N70, 1TZS, 5MLG, 5MKT, 5NFG, 3D91, 2I4Q, 5T4S, 3PSG, 2PSG, 5PEP, 1PSA, 1F34, 4AA9, 3PEP, 4PEP, 1FLH, 1G0V, 1DP5, 2JXR, 1B5F, 3OAD, 3F9Q, 1M43, 5YIA, 5YID, 1SME, 1LF3, 5BWY, 1PFZ, 3QRV, 2BJU, 1QS8, 2ANL, 1LS5, 1MIQ, 1UH7, 3LIZ, 3FNS, 1YG9, 4RLD, 3QVC, 1LYW, 4OBZ, 3ZLQ, 2EWY, 3ZKM, 6FGY, 2QZL, 1TQF, 3EXO, 3QI1, 2Q11, 3TPJ, 1WKR, 1FKN, 2QK5, 3KMX, 3DM6, 2HM1, 2ZJN, 3IXK, 4DPF, 3L58, 2ZHR, 2FDP, 4TRZ, 4B1D, 1SGZ, 3UDH, 3U6A, 5HTZ, 2ZJK, 2OF0, 3VV6, 3CIB, 5MBW, 1YM2, 1W50, 4L7G, 2QU2, 3CKP, 3HVG, 5MXD, 5CLM, 1YM4, 2Q15, 2ZJI, 5EZX, 2VIE, 3LPI, 4YBI, 2WJO, 3TPR, 5V0N, 2HIZ, 6EJ2, 6EJ3, 2ZJJ, 2VA5, 4B78, 3BRA, 4B70, 4EWO, 2ZJH, 1MPP, 2QZX, 4YBF, 2RMP, 3R1G, 1HTR, 4OBZ, 1LYA, 3OAD, 3APR, 1PSO, and 1CZI), the sausage was colored from white (similarity score below 0.7) to red (strict identity). The degree of structural conservation is proportional to the thickness of the sausage, which corresponds to the mean root-mean-square deviation (RMSD) per residue between Cα pairs based on structural alignments. Putative disulfide bridges are indicated by a yellow line. All the graphics were prepared using ENDscript v2 [27] and PyMOL v1.7.2.1.