Abstract
BACKGROUND
Giant cell hepatitis in the adult population remains very poorly defined with only 100 case reports published in the literature over the last three decades.
AIM
To present our center’s experience in an attempt to learn about the predisposing factors, outcomes and efficacy of proposed therapeutic interventions for giant cell hepatitis.
METHODS
A retrospective chart review was conducted through the electronic records of the University of Pittsburgh Medical Center. We queried 36726 liver biopsy reports from January 1, 1991 to December 6, 2016. Our search yielded 50 patients who were identified as carrying a definite diagnosis of post-infantile giant cell hepatitis (PIGCH) by pathology. The data collected included demographic information, laboratory data (liver function tests, autoimmune markers) and transplant status. In order to better analyze patient characteristics and outcomes, subjects were separated into a non-transplant (native) liver group and a post-liver transplant (allograft) group.
RESULTS
The incidence of PIGCH was approximately 0.14% of all biopsies queried in the 25-year period. The mean age was 48 years with 66% females. Liver function tests were classified as 38.2% cholestatic, 35.3% hepatocellular and 26.5% mixed. Autoimmune hepatitis was found to be the most prevalent predisposing factor leading to PIGCH constituting 32% of cases. Management consisted mainly of immunosuppression, viral targeted therapy, supportive care and in six cases liver transplantations.
CONCLUSION
The diagnosis of PIGCH remains clinically challenging and requires a high index of suspicion as well as a thorough history, physical examination, serological workup and liver biopsy. Treatment of the underlying cause can result in clinical stability in a large number of cases.
Keywords: Post-infantile giant cell hepatitis, Liver transplantation, Autoimmune hepatitis
Core tip: Post-infantile giant cell hepatitis is a rare disorder and very poorly defined in the literature. Our study aimed to present our center’s experience in an attempt to shed more light about the predisposing factors, outcomes and efficacy of proposed therapeutic interventions for post-infantile giant cell hepatitis.
INTRODUCTION
Giant cell hepatitis (GCH) is a relatively common histologic finding in neonates. It is believed to occur secondary to insults to immature hepatocytes. In children, it typically presents with cholestasis, conjugated hyperbilirubinemia and variable degrees of inflammation[1]. Idiopathic GCH refers to these histologic findings with a structurally intact biliary system as opposed to conditions where biliary abnormalities are present, such as biliary atresia[1]. The most commonly proposed patho-physiological hypothesis to account for the presence of giant cells includes an ineffective cytoplasmic division in the setting of cellular fission (endomitosis) in contrast to cellular hepatocyte fusion secondary to hepatic injury[2].
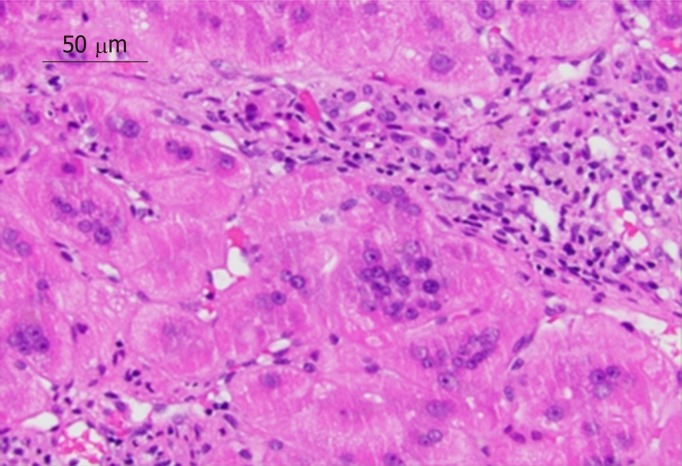
As common as GCH is in children, it is exceedingly rare in adults. GCH in the adult population remains very poorly defined with only 100 case reports published in the literature over the last three decades[3]. In adults the entity is referred to as post-infantile giant cell hepatitis (PIGCH), also known as syncytial or adult onset GCH. PIGCH represents a histologic diagnosis that has been associated with a myriad of medical conditions including infectious, hematologic, autoimmune disorders and drug reactions (Table 1)[3-13]. Pathological analysis is characterized by the presence of giant multinucleated syncytial hepatocytes. In particular, more than four to five nuclei in hepatocytes should be seen in a single lobule combined with other features of hepatitis such as lobular disarray, inflammation, Kupffer cell hypertrophy and spotty hepatocytes necrosis (Figure 1).
Table 1.
Reported causes of post-infantile giant cell hepatitis
| Infectious | Hepatitis A, B, C |
| Epstein-Barr virus (EBV) | |
| Cytomegalovirus (CMV) | |
| Paramyxo-like virus | |
| Human immunodeficiency virus (HIV) | |
| Herpesvirus 6A | |
| Human papillomavirus (HPV) | |
| Autoimmune | Autoimmune hepatitis (AIH) |
| Ulcerative colitis (UC) | |
| Primary sclerosing cholangitis (PSC) | |
| Primary biliary cholangitis (PBC) | |
| Systemic lupus erythematosus (SLE) | |
| Rheumatoid arthritis (RA) | |
| Polyarteritis nodosa (PAN) | |
| Drugs | Methotrexate |
| 6 mercaptopurine | |
| Amytriptyline | |
| P-aminosalicylic acid | |
| Vinyl chloride | |
| Chropromazine | |
| Methotrexate | |
| Hematologic | Chronic lymphocytic leukemia (CLL) |
| Lymphoma | |
| Sickle cell disease (SCC) | |
| Hypereosinophilia | |
| Autoimmune hemolytic anemia | |
| Endocrine | Hypoparathyroidism |
| Infiltrative | Sarcoidosis |
| Post-transplant | - |
| Idiopathic | - |
Figure 1.
Liver biopsy of 44-year-old female with autoimmune hepatitis (hematoxylin and eosin stain 40 ×). Biopsy revealed chronic hepatitis with prominent giant multinucleated hepatocytes.
The clinical course of patients with giant cells on histology is widely variable, ranging from minimal symptoms without major clinical implications to acute liver failure that is often times fatal despite standard clinical care. In the current study, we aimed to present our center’s experience with this very rare disease entity in an attempt to shed more light about its predisposing factors, outcomes and efficacy of proposed therapeutic interventions.
MATERIALS AND METHODS
After obtaining local institutional review board approval, we queried liver biopsy reports (36726) at the University of Pittsburgh Medical Center electronic records using the keywords “giant cell hepatitis” from January 1, 1991 to December 6, 2016. Our search yielded 127 individual patient records, of which 45 were diagnosed prior to 18 years of age. The remaining 82 records were evaluated by three physicians (BM, SM, MM) after which 50 patients were identified as carrying a definite diagnosis of PIGCH based on liver biopsy. In order to better analyze patient characteristics and outcomes, subjects were separated into a non-transplant (native) liver group and a post-liver transplant (allograft) group.
RESULTS
The incidence of PIGCH was approximately 0.14% of all biopsies queried in the 25-year period. The mean age of the studied patient sample was 48 years with 66% females. Liver function tests were classified as follows: 38.2% cholestatic, 35.3% hepatocellular and 26.5% mixed; 73.5% of patients had bilirubin values exceeding 1.5 mg/dL at the time of diagnosis and 42% of patients had bilirubin values exceeding 5 mg/dL. Mean follow up of the entire cohort was over six years (79 mo; SD = 76.1). Patient demographics and liver function tests for patients are outlined in Table 2. Patients with GCH found in the native liver group were older, had higher aspartate aminotransferase, alanine aminotransferase and total bilirubin when compared to the allograft group.
Table 2.
Patient characteristics and liver function tests
| GCH on native liver, n = 40 | GCH on allograft, n = 10 | |
| Mean age in yr | 50.4 | 43.4 |
| Gender | ||
| Male | 14 (35%) | 4 (40%) |
| Female | 26 (65%) | 6 (60%) |
| AST | 433 ± 486 | 175 ± 158 |
| ALT | 488 ± 537 | 232 ± 206 |
| Alkaline phosphatase | 197 ± 151 | 296 ± 197 |
| GGT | 287 ± 582 | 246 ± 182 |
| Bilirubin | 10.9 ± 10.4 | 3.1 ± 3.8 |
GCH: Giant cell hepatitis; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: γ-Glutamyl transpeptidase.
Autoimmune hepatitis (AIH) was found to be the most prevalent predisposing factor leading to PIGCH constituting 32% of cases, while drugs accounted for 12% of cases. Other etiological associations included viral infections [hepatitis A, B, C (HCV), cytomegalovirus (CMV), Epstein-Barr virus], systemic autoimmune conditions (but not enough to give a diagnosis of AIH) and hematologic conditions. In nearly 1/3 of cases, no predisposing factor for PIGCH was found (idiopathic). In the post-transplant population, the most prevalent predisposing factor leading to PIGCH was AIH as well, accounting for 30% of cases.
Autoimmune markers related to liver disease were common: Anti-nuclear antibody in 34% of cases, elevated immunoglobulin G in 22% of cases, anti-smooth muscle antibody in 10% of cases, anti-mitochondrial antibody in 8% of cases and anti-liver kidney microsomal antibody in 2% of cases.
Drugs which were identified as the possible culprit for GCH development consisted of microdantin, ranitidine, omeprazole, moxifloxacin, ranitidine, plaquenil as well as chromium picolinate.
Notable pathological findings included diffuse necrosis in 24% of the patients, inflammation and acute hepatitis in 56% of patients and overt cirrhosis in 12% of patients. Of the ten patients with GCH post-liver transplant, five had concomitant features of acute cellular rejection.
Management of PIGCH consisted mainly of immunosuppression, viral targeted therapy, supportive care and in six cases liver transplantation. Management and outcomes are outlined in Table 3. Among the patients who were treated with immunosuppression, eight patients (53%) had improvement in their liver function tests. Of the patients treated with ganciclovir, two patients (100%) had improvement in their liver function tests.
Table 3.
Predisposing factors, n (%)
| Predisposing factors | GCH on native liver | GCH on allograft |
| AIH | 13 (32) | 3 (30) |
| Drug induced | 6 (15) | 0 |
| No factor identified | 12 (30) | 3 (30) |
| UC | 2 (5) | 3 (30) |
| PSC | 3 (7) | 1 (10) |
| HCV | 2 (5) | 1 (10) |
| CMV | 1 (2) | 1 (10) |
| SLE | 2 (5) | 0 |
| Lymphoma | 2 (5) | 0 |
| HAV | 1 (2) | 0 |
| HBV | 1 (2) | 0 |
| EBV | 1 (2) | 0 |
| Sjogren | 1 (2) | 0 |
| Autoimmune hemolytic anemia | 1 (2) | 0 |
| CLL | 1 (2) | 0 |
| Peripheral eosinophilia | 1 (2) | 0 |
| SCC | 1 (2) | 0 |
| Celiac disease | 1 (2) | 0 |
GCH: Giant cell hepatitis; AIH: Autoimmune hepatitis; UC: Ulcerative colitis; PSC: Primary sclerosing cholangitis; HCV: Hepatitis C virus; CMV: Cytomegalovirus; SLE: Systemic lupus erythematosus; HAV: Hepatitis A virus; HBV: Hepatitis B virus; EBV: Epstein-Barr virus; CLL: Chronic lymphocytic leukemia; SCC: Sickle cell disease.
Among the native group, five patients (13%) required liver transplantation, and one patient developed graft failure from post-transplant GCH and required a second transplant. Five (13%) patients died from liver-related complications in the native liver group compared to two (20%) in the allograft group. Among these seven patients, five died with acute liver failure. Patient #1 had received two liver transplants. The first transplant was for HCV cirrhosis and subsequently developed PIGCH in the allograft despite achieving a sustained virologic response after anti-viral therapy. This patient eventually developed allograft cirrhosis attributed to PIGCH and required a second transplant for this reason. The patient died of a spontaneous intracranial hemorrhage. Patient #2 had developed cirrhosis attributed to PIGCH and died of pneumonia and sepsis. The five remaining patients presented with acute liver failure. Patient #3 was urgently transplanted but developed infected necrotizing pancreatitis to which he succumbed. Patient #4 was found to develop a pneumothorax and died from hemothorax after placement of a thoracotomy tube. Patient #5 died after developing subcapsular hepatic bleeding following a liver biopsy. Patients #6 and #7 developed a massive variceal bleed and lower gastrointestinal bleeding (exact cause unknown), respectively, that led to their demise.
Of the 50 patients with GCH, 12 (6 native and 6 allograft) underwent a repeat liver biopsy of which 66% still had evidence of GCH despite treatment. Half of these patients had undergone liver transplantation (AIH, primary sclerosing cholangitis/AIH overlap, HCV, GCH, alcoholic and cryptogenic cirrhosis). These patients had persistent GCH on repeat biopsies despite immunosuppression. The patient with primary sclerosing cholangitis/AIH overlap had improvement of GCH findings on subsequent biopsy. One subject had evidence of acute cellular then chronic rejection on subsequent biopsies. Cirrhosis developed in a patient transplanted for alcoholic cirrhosis and GCH. Among the native liver group, six patients had recurrent GCH on biopsy. One had acute hepatitis B, while the rest did not have a specific predisposing factor.
DISCUSSION
With only 100 cases reported in the adult literature, PIGCH remains poorly understood. The prevalence of this disease has been reported at 0.1% to 0.25%[3], which is consistent with the incidence in our cohort (0.14%). Given the rarity of this entity, outcomes and management are largely based on anecdotal evidence. There are no approved therapies and no consensus on management strategies[13].
The histological finding of giant cells in adults seems to be a manifestation of hepatic stress as opposed to a primary hepatic injury[3,10]. The diagnosis is made based on the presence of multinucleated giant cells usually evident in zones 1 and 3 of the Rappaport acinus. More than four to five nuclei in hepatocytes should be seen in a single lobule combined with other features of hepatitis such as lobular disarray, acinar inflammation, Kupffer cell hypertrophy and spotty hepatocytes necrosis. Other common features may include non-suppurative cholangitis, ductopenia and different stages of periportal fibrosis leading to cirrhosis[6,14]. Similar histological findings were observed among our patient cohort: The majority had notable inflammation on pathology, while a quarter of them exhibited evidence of hepatic necrosis (28% spotty necrosis, 48% bridging/confluent necrosis, 19% sub-massive necrosis and 5% massive necrosis), with 12% demonstrating overt cirrhosis, which is comparable to previous reported rates in the literature of about 13%[3].
Out of the six liver transplant recipients for PICGH, two died with recurrent disease. The first patient died in the early post-transplant period, and the second patient died 11 years later. Two patients required two more liver transplants each for recurrent decompensated cirrhosis despite being on standard immunosuppression. One patient developed cirrhosis with features of chronic rejection, which was thought to be related to recurrent hepatitis C, and another was related to CMV hepatitis.
Scant data exists on PIGCH in the post-transplant setting with prior observations indicating the need for re-transplantation in the majority of recipients due to recurrent disease. Pappo et al[15] examined the clinical and pathologic course of seven patients who developed GCH after liver transplantation. Five of these patients had GCH as their native liver disease. Two patients died. Two patients required re-transplantation because of recurrent GCH. One patient with recurrent GCH was still alive six years after transplantation. Similarly, in our study, ten patients developed GCH after liver transplantation. Two patients had GCH as their native liver disease. One patient died due to sepsis related to a second liver transplantation. Two patients developed recurrent GCH on the allograft; one of those patients had their immunosuppression increased and had survived at two years and the other patient required re-transplantation. Two patients developed de novo GCH that required an increase in immunosuppression; one patient eventually needed liver transplantation and the other one improved with medical management. One patient developed de novo GCH and CMV hepatitis and was treated with ganciclovir. The remaining four patients were lost to follow up.
Management strategies to treat recurrence mainly consisted of increasing immunosuppression and in rare cases the institution of ribavirin with variable success[16,17].
Our results were consistent with prior reports indicating a potential autoimmune link to the findings of PIGCH. We concluded that an autoimmune type hepatitis was seen in 1/3 of our patients; 34% of the patients had a positive anti-nuclear antibody, 22% had an elevated immunoglobulin G, while 12 patients would fulfill at least a probable diagnosis of AIH based on the AIH scoring system[18] (Table 4).
Table 4.
Management and outcomes, n (%)
| GCH on native liver, n = 40 | GCH on allograft, n = 10 | |
| Management | ||
| Immunosuppression | 11 (28) | 4 (40) |
| Supportive care | 10 (25) | 0 (0) |
| Liver transplantation | 5 (13) | 1 (10) |
| Ganciclovir | 1 (3) | 1 (10) |
| Unknown | 13 (33) | 4 (40) |
| Outcomes | ||
| Survived | 25 (63) | 4 (40) |
| Died | 5 (13) | 2 (20) |
| Unknown | 10 (25) | 4 (40) |
GCH: Giant cell hepatitis.
The majority of our patients were female (66%), which is somewhat different to previous reports with approximately equal numbers between genders[3]. Idiopathic PIGCH was present in 30% of our cohort, which is much higher than prior published studies. A higher incidence of idiopathic PIGCH in our cohort compared to the published literature is likely a manifestation of publication bias, i.e. cases of PICGH where there is no clear link may be less apt to be reported[3]. Drug induced liver injury was the culprit in 12% of cases with all of the reported drugs being novel associations with PIGCH (Table 4).
Viral causes amongst our cohort seem to have been less frequent than previously reported. Outcomes of those with a viral cause was variable, although the cases where CMV infection was felt to be the culprit did respond well to ganciclovir, similar to cases reported in the literature[7,19].
The majority of deaths were in the group labeled idiopathic PIGCH, while only two out of sixteen patients with autoimmune like features died. Notably, all of the idiopathic patients were managed supportively while most of the autoimmune cases were managed with immunosuppression. One patient who died had chronic HCV in addition to AIH. HCV therapy (standard of therapy at the time was interferon-based treatment) was not offered given the patient’s decompensated state. PIGCH has been described in both acute and chronically infected HCV patients (or co-infected with HIV) with a relatively good prognosis after treatment with interferon and ribavirin or immunosuppressive therapy when autoimmune features are present[19-22]. No studies have been published to date using the highly potent direct acting antivirals that might potentially prove to have even better outcomes with higher rates of viral eradication[23].
The presentations and outcomes of our patients coincide with previously reported observations in the literature of being highly variable. Some patients only manifested in mild elevations in liver function tests while others developed acute liver failure resulting in death or the need for liver transplantation (Table 2). Most patients responded well to immunosuppressive therapy that mainly consisted of intravenous hydrocortisone, prednisone, azathioprine and tacrolimus, especially with the presence of autoimmune features. One case (previously published) with PIGCH secondary to AIH complicating ulcerative colitis responded to prednisone with improved liver functions despite worsening ulcerative colitis (the patient ultimately required a colectomy)[12]. Several cases of PIGCH associated with chronic lymphocytic leukemia have been reported with largely favorable outcomes after being managed with intravenous immunoglobulins (in the events where immunoglobulins are low), rituximab or steroids[8,13]. This is similar to our patient with chronic lymphocytic leukemia who was managed successfully with prednisone but ultimately developed cirrhosis[24].
Our study has several limitations. It is based on retrospective chart review and is mainly descriptive. That being said, it includes the largest number of unique cases of PIGCH from a single institution included in a single manuscript.
The exact etiology of PIGCH and mechanism of injury remains unknown, and the histological findings are likely related to an idiosyncratic or cytopathic response to various hepatocyte stimuli. Our series suggested an autoimmune cause as the most common association. The diagnosis of PIGCH remains clinically challenging and requires a high index of suspicion as well as a thorough history, physical examination and serological workup, which should include viral, hematologic and autoimmune causes. Ultimately a liver biopsy is required as PICGH remains a purely histomorphological diagnosis. Treatment of the underlying cause (especially if it is autoimmune or viral) can result in clinical stability in a large number of cases. Treatment and monitoring should be done in close association with specialty centers including those capable of liver transplantation.
ARTICLE HIGHLIGHTS
Research background
Giant cell hepatitis in the adult population remains very poorly defined with only 100 case reports published in the literature over the last three decades. Pathological analysis is characterized by the presence of giant multinucleated syncytial hepatocytes. The clinical course of patients with giant cells on histology is widely variable, ranging from minimal symptoms without major clinical implications to acute liver failure that is often times fatal despite standard clinical care.
Research objectives
Our primary objective was to present our center’s experience in an attempt to learn about the predisposing factors, outcomes and efficacy of proposed therapeutic interventions for giant cell hepatitis.
Research methods
A retrospective chart review was conducted through the electronic records of the University of Pittsburgh Medical Center. We queried 36726 liver biopsy reports from January 1, 1991 to December 6, 2016. Our search yielded 50 patients who were identified as carrying a definite diagnosis of post-infantile giant cell hepatitis (PIGCH) by pathology. The data collected included demographic information, laboratory data (liver function tests, autoimmune markers) and transplant status. In order to better analyze patient characteristics and outcomes, subjects were separated into a non-transplant (native) liver group and a post-liver transplant (allograft) group.
Research results
The incidence of PIGCH was approximately 0.14% of all biopsies queried in the 25-year period. The mean age was 48 years with 66% females. Liver function tests were classified as 38.2% cholestatic, 35.3% hepatocellular and 26.5% mixed. Autoimmune hepatitis was found to be the most prevalent predisposing factor leading to PIGCH constituting 32% of cases. Management consisted mainly of immunosuppression, viral targeted therapy, supportive care and in six cases liver transplantation.
Research conclusions
The diagnosis of PIGCH remains clinically challenging and requires a high index of suspicion as well as a thorough history, physical examination, serological workup and liver biopsy. Treatment of the underlying cause can result in clinical stability in a large number of cases.
Research perspectives
This study reports our center’s experience with PIGCH and the importance of thorough history, physical examination, serologic work up and liver biopsy in its diagnosis. Further research should aim at recognizing risk factors for progression from PIGCH to liver failure and further evaluation of therapeutic interventions (immunosuppression vs viral targeted therapy vs liver transplantation).
Footnotes
Institutional review board statement: This study was reviewed and approved by the University of Pittsburgh Medical Center Institution Review Board PRO12030073.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: There were no conflict of interests to disclose.
Manuscript source: Unsolicited manuscript
Peer-review started: May 17, 2019
First decision: July 4, 2019
Article in press: October 18, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A, Pop TL, Rubbini M S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Liu MY
Contributor Information
Bassem Matta, Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh, Pittsburgh, PA 15213, United States.
Ricardo Cabello, Department of Internal Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, United States.
Mordechai Rabinovitz, Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh, Pittsburgh, PA 15213, United States.
Marta Minervini, Department of Pathology, University of Pittsburgh, Pittsburgh, PA 15213, United States.
Shahid Malik, Department of Medicine, University of Pittsburgh, Division of Gastroenterology Hepatology and Nutrition, Pittsburgh, PA 15213, United States. maliks@upmc.edu.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymized data may be granted following review.
References
- 1.Torbenson M, Hart J, Westerhoff M, Azzam RK, Elgendi A, Mziray-Andrew HC, Kim GE, Scheimann A. Neonatal giant cell hepatitis: histological and etiological findings. Am J Surg Pathol. 2010;34:1498–1503. doi: 10.1097/PAS.0b013e3181f069ab. [DOI] [PubMed] [Google Scholar]
- 2.Correa KK, Nanjundiah P, Wirtschafter DD, Alshak NS. Idiopathic neonatal giant cell hepatitis presenting with acute hepatic failure on postnatal day one. J Perinatol. 2002;22:249–251. doi: 10.1038/sj.jp.7210670. [DOI] [PubMed] [Google Scholar]
- 3.Bihari C, Rastogi A, Sarin SK. Postinfantile giant cell hepatitis: an etiological and prognostic perspective. Hepat Res Treat. 2013;2013:601290. doi: 10.1155/2013/601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinra P, John BM. Hepatitis - A induced Non Infantile Giant Cell Hepatitis. Med J Armed Forces India. 2007;63:182–183. doi: 10.1016/S0377-1237(07)80073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SJ, Mathew J, MacSween RN, Bennett MK, Burt AD. Post-infantile giant cell hepatitis: histological and immunohistochemical study. J Clin Pathol. 1994;47:1022–1027. doi: 10.1136/jcp.47.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Protzer U, Dienes HP, Bianchi L, Lohse AW, Helmreich-Becker I, Gerken G, Meyer zum Büschenfelde KH. Post-infantile giant cell hepatitis in patients with primary sclerosing cholangitis and autoimmune hepatitis. Liver. 1996;16:274–282. doi: 10.1111/j.1600-0676.1996.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 7.Falasca L, Nonno FD, Palmieri F, Licordari R, Iannicelli G, Antonucci G, Baiocchini A. Two cases of giant cell hepatitis in HIV-infected patients. Int J STD AIDS. 2012;23:e3–e4. doi: 10.1258/ijsa.2009.009407. [DOI] [PubMed] [Google Scholar]
- 8.Gupta E, Yacoub M, Higgins M, Al-Katib AM. Syncytial giant cell hepatitis associated with chronic lymphocytic leukemia: a case report. BMC Blood Disord. 2012;12:8. doi: 10.1186/1471-2326-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouguila J, Mabrouk S, Tilouche S, Bakir D, Trabelsi A, Hmila A, Boughammoura L. Giant cell hepatitis with autoimmune hemolytic anemia in a nine month old infant. World J Hepatol. 2013;5:226–229. doi: 10.4254/wjh.v5.i4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estradas J, Pascual-Ramos V, Martínez B, Uribe M, Torre A. Autoimmune hepatitis with giant-cell transformation. Ann Hepatol. 2009;8:68–70. [PubMed] [Google Scholar]
- 11.Moreno-Otero R, Trapero-Marugán M, García-Buey L, García-Sancchez A. Drug-induced postinfantile giant cell hepatitis. Hepatology. 2010;52:2245–2246. doi: 10.1002/hep.23830. [DOI] [PubMed] [Google Scholar]
- 12.Labowitz J, Finklestein S, Rabinovitz M. Postinfantile giant cell hepatitis complicating ulcerative colitis: a case report and review of the literature. Am J Gastroenterol. 2001;96:1274–1277. doi: 10.1111/j.1572-0241.2001.03711.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, Njei B. Syncytial giant cell hepatitis in a patient with chronic lymphocytic leukemia. J Dig Dis. 2015;16:683–688. doi: 10.1111/1751-2980.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaney K, Goodman ZD, Ishak KG. Postinfantile giant-cell transformation in hepatitis. Hepatology. 1992;16:327–333. doi: 10.1002/hep.1840160208. [DOI] [PubMed] [Google Scholar]
- 15.Pappo O, Yunis E, Jordan JA, Jaffe R, Mateo R, Fung J, Demetris AJ. Recurrent and de novo giant cell hepatitis after orthotopic liver transplantation. Am J Surg Pathol. 1994;18:804–813. doi: 10.1097/00000478-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lerut JP, Claeys N, Ciccarelli O, Pisa R, Galant C, Laterre PF, Palazzo U. Recurrent postinfantile syncytial giant cell hepatitis after orthotopic liver transplantation. Transpl Int. 1998;11:320–322. doi: 10.1007/s001470050151. [DOI] [PubMed] [Google Scholar]
- 17.Durand F, Degott C, Sauvanet A, Molas G, Sicot C, Marcellin P, Belghiti J, Erlinger S, Benhamou JP, Bernuau J. Subfulminant syncytial giant cell hepatitis: recurrence after liver transplantation treated with ribavirin. J Hepatol. 1997;26:722–726. doi: 10.1016/s0168-8278(97)80440-0. [DOI] [PubMed] [Google Scholar]
- 18.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 19.Gábor L, Pál K, Zsuzsa S. Giant cell hepatitis in adults. Pathol Oncol Res. 1997;3:215–218. doi: 10.1007/BF02899924. [DOI] [PubMed] [Google Scholar]
- 20.Moreno A, Moreno A, Pérez-Elías MJ, Quereda C, Fernández-Muñoz R, Antela A, Moreno L, Bárcena R, López-San Román A, Celma ML, García-Martos M, Moreno S. Syncytial giant cell hepatitis in human immunodeficiency virus-infected patients with chronic hepatitis C: 2 cases and review of the literature. Hum Pathol. 2006;37:1344–1349. doi: 10.1016/j.humpath.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Micchelli ST, Thomas D, Boitnott JK, Torbenson M. Hepatic giant cells in hepatitis C virus (HCV) mono-infection and HCV/HIV co-infection. J Clin Pathol. 2008;61:1058–1061. doi: 10.1136/jcp.2008.058560. [DOI] [PubMed] [Google Scholar]
- 22.Tajiri K, Shimizu Y, Tokimitsu Y, Tsuneyama K, Sugiyama T. An elderly man with syncytial giant cell hepatitis successfully treated by immunosuppressants. Intern Med. 2012;51:2141–2144. doi: 10.2169/internalmedicine.51.7870. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Nguyen D, Hu KQ. Chronic Hepatitis C Virus Infection: A Review of Current Direct-Acting Antiviral Treatment Strategies. N Am J Med Sci (Boston) 2016;9:47–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Horsmans Y, Galant C, Nicholas ML, Lamy M, Geubel AP. Failure of ribavarin or immunosuppressive therapy to alter the course of post-infantile giant-cell hepatitis. J Hepatol. 1995;22:382. doi: 10.1016/0168-8278(95)80298-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymized data may be granted following review.