Abstract
Mammography is the primary screening method for breast cancers. However, the sensitivity of mammographic screening is lower for dense breasts, which are an independent risk factor for breast cancers. Automated breast ultrasound (ABUS) is used as an adjunct to mammography for screening breast cancers in asymptomatic women with dense breasts. It is an effective screening modality with diagnostic accuracy comparable to that of handheld ultrasound (HHUS). Radiologists should be familiar with the unique display mode, imaging features, and artifacts in ABUS, which differ from those in HHUS. The purpose of this study was to provide a comprehensive review of the clinical significance of dense breasts and ABUS screening, describe the unique features of ABUS, and introduce the method of use and interpretation of ABUS.
Keywords: Dense breast, Automated breast ultrasound, Screening, Early detection of cancer, Breast cancer
INTRODUCTION
In the eight randomized controlled trials conducted so far, mammography detected breast cancers in the early stages and thereby reduced breast cancer mortality by up to 20%. Mammography is the primary screening method for breast cancers (1,2,3).
The sensitivity of mammographic screening is lower for dense breasts, which are an independent risk factor for breast cancers (4,5). Supplemental breast ultrasound (US) screening is expected to detect mammographically occult breast cancers (6). With the increasing demands of supplemental US screening, radiologists are unable to widely use handheld US (HHUS) because of limited human resources and heavy workload. Automated breast US (ABUS) has been developed to overcome the limitations of operator dependency and lack of reproducibility in HHUS, and it is time-efficient for radiologists (7,8,9,10). ABUS has been approved in the United States and Europe as an adjunct to mammography for screening, especially for asymptomatic women with dense breasts (8,10).
In this study, we addressed the clinical significance of dense breasts and effectiveness of ABUS screening of breast cancers for women with dense breasts. In addition, we introduced the method of use and interpretation of ABUS. The reader will gain comprehensive knowledge of the effective applications and unique imaging features of ABUS for women with dense breasts.
Dense Breasts
Dense breasts are associated with low mammographic sensitivity and breast cancer development. The frequency of dense breasts in the screening population over the age of 40 years was 43.3% in the United States and 54.8% in Korea. Moreover, among young women in their 40s, it increased to 56% and 83.2%, respectively (11,12).
Presence of dense breast tissue may lead to reduction in the sensitivity of mammography. According to the Breast Cancer Surveillance Consortium reports, the sensitivity of mammography decreased from 85.7–88.8% in patients with breast tissue composed almost entirely of fatty tissue (non-dense breast tissue) to 62.2–68.1% in patients with extremely dense breast tissues (13).
Breast density is an independent risk factor for breast cancers (13,14). Dense breasts are in the intermediate risk category for breast cancers (lifetime risk: 15–20%) (1). Women with breast density ≥ 75% had 4–6 times greater risk for developing breast cancers compared to women with breast density ≤ 10% (15,16), and women with breast density of 50–74% had 2.9 times greater risk compared to women with breast density ≤ 10% (17). Park et al. (18) reported increased risks for breast cancer with greater breast densities in Korean women. Compared to women with breasts composed almost entirely of fatty tissue, women with extremely dense breasts had a five times higher risk of breast cancer, and women with heterogeneously dense breasts had a 3.8 times higher risk (18).
There is insufficient evidence for reduction in mortality with US screening, so no recommendations have been established for the screening guidelines. However, in the United States, legislative changes require healthcare providers to notify women of their breast tissue density and advise supplemental screening to women with dense breasts (19). The American College of Radiology (ACR) states that supplemental US screening is an option for women with dense breasts and supplemental magnetic resonance imaging may be performed depending on risk factors, such as a history of lobular carcinoma in situ in women with intermediate risk for breast cancers (20). The Korean guidelines neither recommend nor oppose US as a screening modality (21).
Effectiveness and Diagnostic Performance of ABUS Screening
Although the evidence on long-term benefits is limited, supplemental US screening has high sensitivity for cancer detection, especially in early-stage invasive cancers, and reduces the frequency of interval cancers (6,22,23).
In several studies, screening ABUS yielded a high diagnostic performance (Table 1), similar to screening HHUS (1,9,24,25,26,27). Supplemental ABUS screening increased breast cancer detection by 1.9–7.7 cases per 1000 women. Sensitivity increased by 21.6–41.0%, but specificity varied. Recall and biopsy rates increased while positive predictive value-3 (PPV3) decreased by 4.2–15.8%. The largest ABUS study additionally detected 1.9 cases of breast cancer per 1000 women (25), which was similar to the results of Japan Strategic Anti-cancer Randomized Trial (J-START) (22) but lower than the results of American College of Radiology Imaging Network 6666 (23) (Table 2). Differences in the cancer detection rate were thought to be because of the different inclusion criteria. The largest ABUS study had a proportion of invasive cancers of 93.3%, mean breast lesion size of 12.9 mm, and proportion of node-negative cancers of 92.6% (25), which were similar to the results of HHUS screening (22,23). ABUS screening was effective in detecting small, invasive, and predominantly node-negative breast cancers, similar to HHUS screening.
Table 1. Diagnostic Performance of Supplemental ABUS Screening.
| Prospective Studies | Population (Numbers) | US Only Detected Cancers | Sensitivity (%) | Specificity (%) | Recall Rate (%) | CDR Per 1000 | Biopsy Rate (%) | PPV3 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US + MMG | MMG | US + MMG | MMG | US + MMG | MMG | US + MMG | MMG | US + MMG | MMG | US + MMG | MMG | |||
| Additional by US | Additional by US | Additional by US | Additional by US | Additional by US | Additional by US | |||||||||
| Kelly et al. (24) | Women with dense breast/at elevated risk (4419) | 23 | 81.0 | 40.0 | 98.7 | 95.1 | 9.6 | 4.2 | 7.2 | 3.6 | N.R. | N.R. | N.R. | N.R. |
| 41.0 | 3.6 | 5.4 | 3.6 | N.R. | N.R. | |||||||||
| Brem et al. (25) | Women with dense breast (15318) | 30 | 100.0 | 73.2 | 72.0 | 85.4 | 28.4 | 15.0 | 7.3 | 5.4 | 7.4 | 3.8 | 9.8 | 14.0 |
| 26.8 | −13.4 | 13.4 | 1.9 | 3.6 | −4.2 | |||||||||
| Wilczek et al. (26) | Women with dense breast (1688) | 4 | 100.0 | 63.6 | 98.4 | 99.0 | 2.2 | 1.3 | 6.6 | 4.2 | 1.3 | 0.6 | 47.8 | 63.6 |
| 36.4 | −0.6 | 0.9 | 2.4 | 0.7 | −15.8 | |||||||||
| Giuliano et al. (27) | Women with dense breast (3418 test/4076 control) | N.R. | 97.6 | 76.0 | 99.7 | 98.2 | N.R. | N.R. | 12.3 | 4.6 | N.R. | N.R. | N.R. | N.R. |
| 21.6 | 1.5 | N.R. | 7.7 | N.R. | N.R. | |||||||||
ABUS = automated breast ultrasound, CDR = cancer detection rate, MMG = mammography, N.R. = not reported, PPV3 = positive predictive value-3, US = ultrasound
Table 2. Additional Cancer Detection and Proportion of Invasive Cancers in Supplemental Ultrasound Screening.
| Study | SomoInsignt (25) | J-START (22) | ACRIN 6666 (23) |
|---|---|---|---|
| Modality | ABUS | HHUS | HHUS |
| Study population | Asymptomatic women with dense breast 15318 | Asymptomatic women in their 40's 36752 | Asymptomatic women at high risk 2809 |
| Period | 2009–2011 | 2007–2011 | 2004–2006 |
| Additional cancer detection | 1.9/1000 women | 1.84/1000 women | 5.3/1000 women |
| Proportion of invasive cancer (%) | 93.3 | 82.0 | 93.7 |
| Mean size of invasive cancer (mm) | 12.9 | 14.2 | 10.0 |
| Proportion of node negative cancer (%) | 92.6 | 85.5 | 96.7 |
ACRIN = American College of Radiology Imaging Network, HHUS = handheld ultrasound, J-START = Japan Strategic Anti-cancer Randomized Trial
Interpretation Criteria for ABUS Screening
There are no screening US guidelines applicable worldwide. To date, only one guideline and a few studies on the interpretation and management of screening US have been published (28,29,30,31). J-START adopted screening US guidelines from the Japanese Association of Breast and Thyroid Sonology (JABTS) (22), which was different from ACR breast imaging-reporting and data system (BI-RADS) in the lexicon (28,29) (Table 3). The biggest difference was inclusion of a description of non-mass lesions, which was done only in the JABTS guidelines. Furthermore, the categorization and management were different (Table 3). In the flow chart for assessing masses provided by the JABTS guidelines (Fig. 1), masses without an interrupted interface or echogenic halo were divided based on the size and depth-to-width ratio. JABTS guidelines reflected that lesions smaller than 5 mm had low PPVs. Ban et al. verified the usefulness of this guideline (29).
Table 3. Comparison of Guidelines.
| Mass | JABTS | ACR BI-RADS |
|---|---|---|
| Shape | Oval/round, lobulated, polyponal, irregular | Oval, round, irregular |
| Margin | Well defined–smooth, rough | Circumscribed |
| Indistnct–with/without echogenic halo | Not circumscribed–indistinct, angular, microlobulated, spiculated | |
| Obscure | ||
| Orientation | Small (depth/width ratio < 0.7), large (≥ 0.7) | Parallel, non-parallel |
| Echogenicity | Echolevel–anechoic, hyper-, hypo-, isoechoic | Anechoic, hyper-, hypo-, isoechoic, complex cystic and solid, heterogeneous |
| Homogeneity–heterogeneous, homogeneous | ||
| Non-mass | Ductal dilatations with internal echoes, hypoechoic area in mammary gland, architectural distortion |
ACR = American College of Radiology, BI-RADS = breast imaging-reporting and data system, CNB = core needle biopsy, FNAB = fine needle aspiration biopsy, FU = follow-up, JABTS = Japanese Association of Breast and Thyroid Sonology
Fig. 1. Flow chart provided by Japanese Association of Breast and Thyroid Sonology for assessing mass lesions.
There were two studies validating the detection of BI-RADS category 3 on screening US. The malignancy rate of category 3 lesions was 0.8%, and only 0.1% of the cases had suspicious changes at the 6-month follow-up (30). Multiple bilateral circumscribed masses showed no signs of malignancy in the biopsy specimen or on follow-up US (31). Therefore, we concluded that category 3 lesions, including multiple bilateral circumscribed masses, required a follow-up of 1 year with screening.
A prospective multicenter Korean ABUS screening trial is underway. It aimed to evaluate cancer detection on ABUS alone in asymptomatic women in their 40s. Based on previous studies (30,31), we modified BI-RADS and JABTS guidelines to develop interpretation criteria for ABUS screening (Table 4). Some solid masses assessed as BI-RADS category 3 have been classified under category 2 in this guideline. To date, a total of 846 people were screened with ABUS, and 5 cases of cancer were diagnosed. The recall rate was 7.56%. PPV for biopsy was 27.7%, and the cancer detection rate was 5.9 cases per 1000 women. Interim results were better compared to previous ABUS screening trials (24,25,26,27); however, verification of the interpretation criteria using more follow-up data is required.
Table 4. Interpretation Criteria for ABUS Screening.
| Category | Finding | Size (mm) | Management |
|---|---|---|---|
| 2 | A: Simple cyst/IMN/calcified FA/fat-containing lesion | 1 year FU | |
| B: Multiple, oval, circumscribed complicated cysts or masses | |||
| C: Non-simple cysts in setting of multiple or bilateral cysts (at least three cysts, with at least one in each breast) | |||
| D: Round, circumscribed, solid mass | ≤ 5 | ||
| E: Oval circumscribed, parallel solid mass | ≤ 10 | ||
| 3 | Isolated complicated cyst | 6 months FU | |
| Round circumscribed solid mass | > 5 | ||
| Oval circumscribed parallel mass | > 10 | ||
| Clustered microcysts | |||
| Fat necrosis | |||
| Intraductal well defined lesion | |||
| 4 | Others | Biopsy | |
| 5 | Irregular, spiculated mass | Biopsy |
FA = fibroadenoma, IMN = intramammary lymph node
Wise Use of ABUS
ABUS is technically different from HHUS (Table 5). Comprehensive knowledge of indications for ABUS and technical differences between ABUS and HHUS is essential for its appropriate use.
Table 5. Technical Differences between ABUS and HHUS.
| Techniques | ABUS | HHUS |
|---|---|---|
| 3D view | 3D reconstruction | - |
| FOV (cm) | 15 × 17 | 4–6 × 4–6 |
| Scan direction | Transverse | Transverse, longitudinal, radial, antiradial |
| Probe (MHz) | 5–14 (average 10 MHz) | 5–17, 18 |
| Elastography, color Doppler | - | Available |
| Focal zone | Wide and fixed | Manual setting |
| Coupling agent | Lotion | Gel |
FOV = field of view, 3D = three-dimensional
Indications
The main indication for ABUS screening is the presence of dense breasts in asymptomatic women (8,10). Currently, indications for ABUS in diagnosis remain unclear. However, there are no absolute contraindications (postoperative breasts or breasts with implants) (32). ABUS could document the multiplicity or bilaterality of breast cancers in cases of additionally suspicious lesion detected on magnetic resonance imaging (33). Furthermore, ABUS could help estimate the tumor extent precisely in cases of ductal carcinoma in situ (9,34,35) and monitor variations in tumor dimensions during the course of chemotherapy (9).
Technical Basics
ABUS comprises of a US scanner and special stationary device with a transducer, which moves automatically in a scan box (Fig. 2A). The slice thickness is adjustable from 0.5 mm to 8.0 mm (default value: 0.5 mm), and up to 448 axial slices are acquired.
Fig. 2. ABUS with dedicated scanner and workstation.
A. ACUSON S2000 Automated Breast Volume Scanner (Siemens Healthineers) comprises of scanner and special stationary device with transducer. B. Images are obtained in three to five views per breast. C. Workstation provides three-dimensional reconstruction view, and display mode is chosen from four settings. ABUS = automated breast ultrasound, AP = anteroposterior
The patient lies in a supine position, and ABUS of breasts is performed in anteroposterior, medial, and lateral views routinely and in the superior or inferior view additionally in cases of large breasts (Fig. 2B). Image acquisition in six views takes approximately 10 minutes.
The axial image series is sent to a workstation where three-dimensional (3D) reconstructions of sagittal and coronal images occur. This ABUS-dedicated workstation with a dedicated software package provides an efficient and comprehensive analysis of the 3D data and facilitates easy reporting. The number of images varies with the slice thickness and depth (based on the breast cup size), but approximately 2000 images are usually generated. The display mode is chosen (Fig. 2C). When the cursor is placed on the mass in the axial view, coronal and sagittal views automatically display lesions. The average reading time is approximately 9 minutes (36), and the reading time varies with the presence/absence of abnormalities and display mode (9,37). The storage capacity per patient is about 1 GB, so the representative images, instead of whole images, are selected and sent to a picture archiving and communication system.
Qualified Interpretation of ABUS
ABUS has features that are different from HHUS. Computer-aided detection (CAD) has been introduced as an auxiliary software.
Display Mode and Unique Features of ABUS
The coronal view is the unique display mode of ABUS, which shows the entire breast anatomy. The analysis is fast and comprehensive (37,38). A single-center retrospective study compared the detection rates of coronal and transverse display modes and reported that the transverse view was better than the coronal view for lesion detection (37). However, most screening studies use the coronal view rather than the transverse view. Further studies are needed to verify whether or not the coronal view alone is sufficient.
The retraction phenomenon on ABUS is a sign of malignancy, which presents as a stellate pattern around the lesion (Fig. 3) (35). It showed a sensitivity of 80–89% and specificity of 96–100% for cancer detection (9,35,39). It was best visualized in the coronal view (40) and might be absent in fast-growing cancers (35).
Fig. 3. ABUS image of 58-year-old woman with right breast cancer.
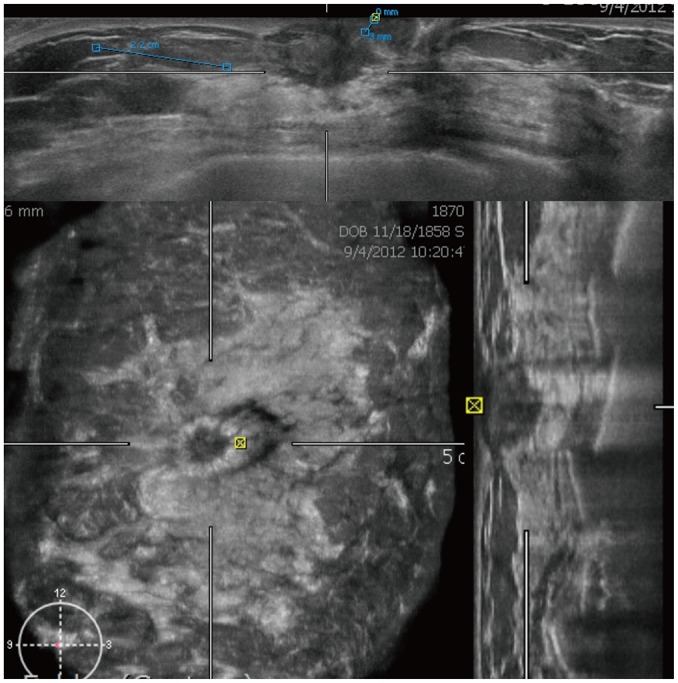
Axial (upper column), coronal (right lower column), and sagittal (left lower column) images show irregular spiculated hypoechoic mass measuring 2.2 cm. Retraction phenomenon is seen in coronal view.
The white-wall sign presents as an echogenic wall in the coronal view and corresponds to the acoustic enhancement on HHUS (Fig. 4). It is mainly seen in benign lesions, such as simple cysts, fibroadenomas, and papillomas, and rarely associated with cancers (9,38).
Fig. 4. ABUS image of 44-year-old woman.

Axial and coronal images show few cysts. White-wall sign is seen in coronal view (arrowheads).
The coronal view had the potential to detect non-mass lesions by depicting dilated ducts and intraductal echoes (Fig. 5). Ductal carcinomas in situ and papillary neoplasms are frequently seen as a non-mass lesion. ABUS in the coronal view allows a more precise evaluation of the lesion extent compared to HHUS (9,34,35).
Fig. 5. ABUS image of 49-year-old woman with atypical papilloma.
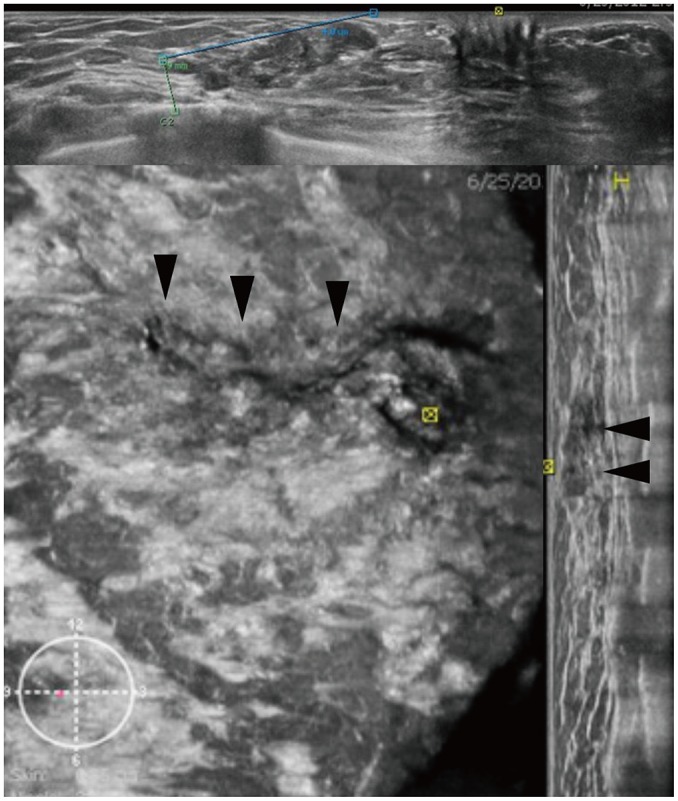
Three orthogonal views reveal segmental non-mass lesion of intraductal masses measuring 4.8 cm (arrowheads).
Artifacts and Image Quality
The optimal image quality should be guaranteed for screening. However, the image quality and ultrasonic resolution diminish with poor contact, marked shadowing due to fibrotic breasts, and artifacts (10,35,41).
The nipple shadow and reverberation artifacts frequently occurred with ABUS (Fig. 6) (10,35). Skip artifacts can be used to detect isoechoic masses. They present as a transverse anechoic line at the location of change in tissue stiffness due to a mass (Fig. 7) (10).
Fig. 6. ABUS image of 46-year-old woman.
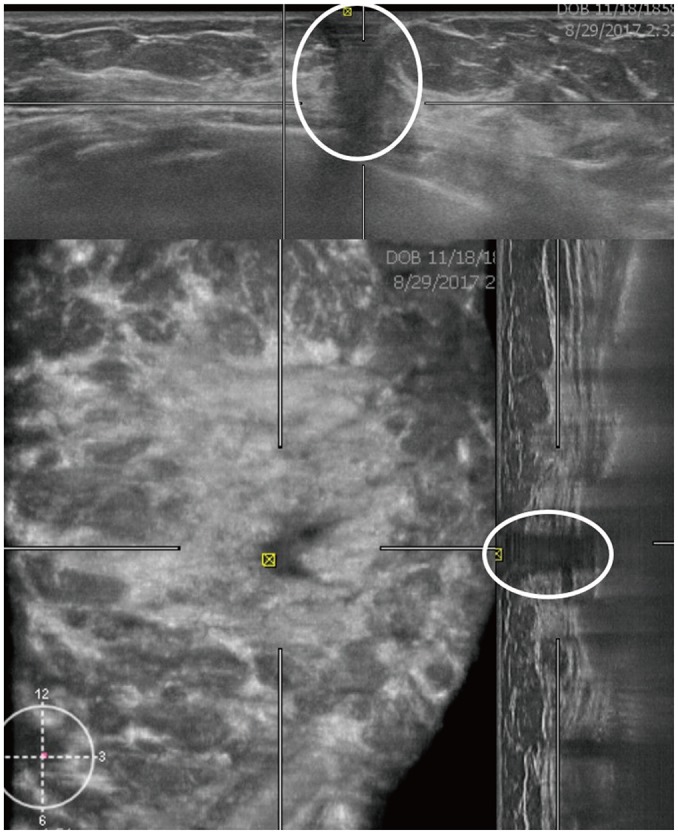
Axial and sagittal images demonstrate nipple shadow, and reverberation artifact is seen as hypoechoic area with shadowing and multiple parallel echogenic lines (circles). This was interpreted as space occupying lesion, but handheld ultrasound showed no focal lesions (not shown). Therefore, this is false-positive case.
Fig. 7. ABUS image of 28-year-old woman with fibroadenoma.
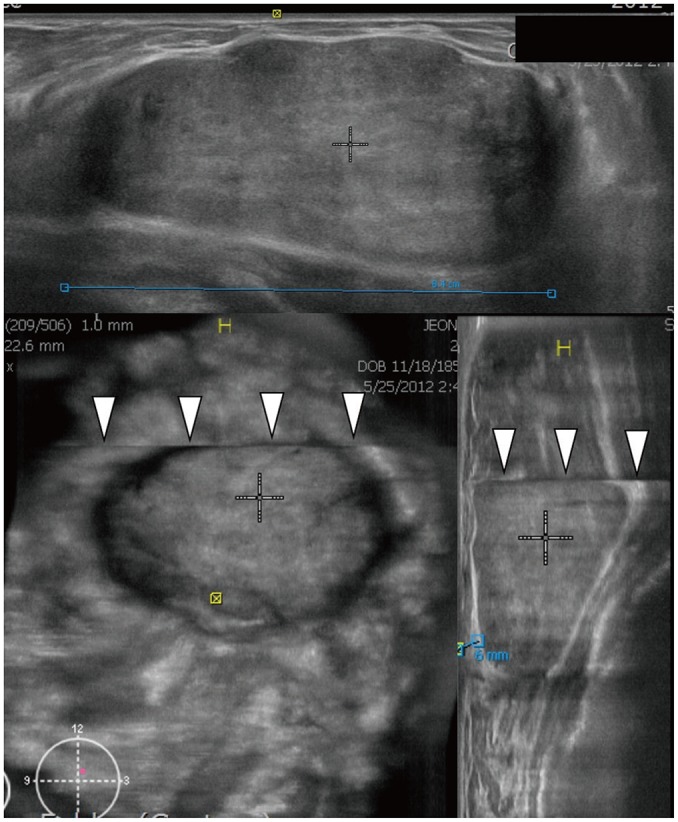
Three orthogonal views show circumscribed oval hypoechoic mass measuring 8.2 cm. Coronal and sagittal images demonstrate skip artifact as transverse line (arrowheads) above mass.
The training and experience of radiologists and technicians play an important role in obtaining high-quality images, resulting in qualified interpretation.
CAD Application
CAD improves the radiologist's diagnostic performance and shortens the reading time. The ABUS-dedicated CAD software improves the radiologist's performance, and the area under the receiver operating characteristic curve (AUC) has increased from 0.77–0.82 without CAD to 0.84 with CAD (42,43). A study showed improved sensitivity with CAD for all tested readers (42). In another study, CAD significantly improved AUC only for radiologists without experience in ABUS interpretation (44). CAD significantly shortened the reading time in all studies (49.3 seconds without CAD and 44.7 seconds with CAD (44); 158.3 seconds without CAD and 133.4 seconds with CAD (45), and 3 minutes 33 seconds without CAD and 2 minutes 24 seconds with CAD (43)). A CAD software (QVCAD, Qview Medical Inc., Los Altos, CA, USA) was used in a few studies (42,43,44,45). This system employs several image pattern recognition processes and artificial neural networks to detect suspicious areas measuring 5 mm or more in diameter (Fig. 8).
Fig. 8. ABUS image of 53-year-old woman with left breast cancer.
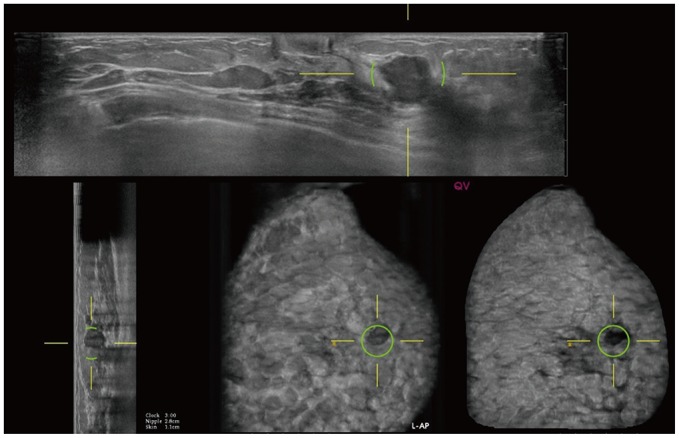
Computer-aided detection with QVCAD (Qview Medical Inc.) system shows cancer and marks suspicious mass with green circles.
Limitations of ABUS Screening
ABUS screening is also limited by its high recall rate and biopsy rate with low PPV, similar to HHUS screening (9,24,25,26). Screening US guidelines are required to reduce the frequency of false-positive results and improve PPV. In addition, a certain period of learning time is required to achieve the desirable PPV (46).
Biopsy methods under ABUS guidance have not been developed, so HHUS is performed in another step to re-examine the patients (9).
SUMMARY
ABUS is an effective screening modality to detect mammographically occult breast cancers in women with dense breasts. The coronal view is a unique display mode with high diagnostic accuracy. CAD helps detect breast cancers and reduce the interpretation time. Efforts should be made to reduce the hazards of ABUS screening, and further studies are required to verify the cost-effectiveness of ABUS for supplemental screening in women with dense breasts.
Footnotes
This study was supported by a grant from the Korean Society of Breast Imaging.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Niell BL, Freer PE, Weinfurtner RJ, Arleo EK, Drukteinis JS. Screening for breast cancer. Radiol Clin North Am. 2017;55:1145–1162. doi: 10.1016/j.rcl.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update From the American Cancer Society. JAMA. 2015;314:1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH. A systematic review on radiologists' knowledge of breast cancer screening. J Korean Soc Radiol. 2019;80:8–18. [Google Scholar]
- 4.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13:223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajaram N, Mariapun S, Eriksson M, Tapia J, Kwan PY, Ho WK, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat. 2017;161:353–362. doi: 10.1007/s10549-016-4054-y. [DOI] [PubMed] [Google Scholar]
- 6.Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204:234–240. doi: 10.2214/AJR.13.12072. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Kang BJ, Choi BG, Choi JJ, Lee JH, Song BJ, et al. Radiologists' performance for detecting lesions and the interobserver variability of automated whole breast ultrasound. Korean J Radiol. 2013;14:154–163. doi: 10.3348/kjr.2013.14.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan SS. Automated whole breast ultrasound. Radiol Clin North Am. 2014;52:539–546. doi: 10.1016/j.rcl.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Rella R, Belli P, Giuliani M, Bufi E, Carlino G, Rinaldi P, et al. Automated breast ultrasonography (ABUS) in the screening and diagnostic setting: indications and practical use. Acad Radiol. 2018;25:1457–1470. doi: 10.1016/j.acra.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH. Image quality and artifacts in automated breast ultrasonography. Ultrasonography. 2019;38:83–91. doi: 10.14366/usg.18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Lee EH, Jun JK, Shin DR, Park YM, Kim HW, et al. Alliance for Breast Cancer Screening in Korea (ABCS-K) Analysis of participant factors that affect the diagnostic performance of screening mammography: a report of the Alliance for Breast Cancer Screening in Korea. Korean J Radiol. 2017;18:624–631. doi: 10.3348/kjr.2017.18.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freer PE. Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics. 2015;35:302–315. doi: 10.1148/rg.352140106. [DOI] [PubMed] [Google Scholar]
- 14.Sprague BL, Conant EF, Onega T, Garcia MP, Beaber EF, Herschorn SD, et al. PROSPR Consortium. Variation in mammographic breast density assessments among radiologists in clinical practice: a multicenter observational study. Ann Intern Med. 2016;165:457–464. doi: 10.7326/M15-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. Mammographic breast density and breast cancer risk: interactions of percent density, absolute dense, and non-dense areas with breast cancer risk factors. Breast Cancer Res Treat. 2015;150:181–189. doi: 10.1007/s10549-015-3286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 17.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 18.Park B, Cho HM, Lee EH, Song S, Suh M, Choi KS, et al. Does breast density measured through population-based screening independently increase breast cancer risk in Asian females? Clin Epidemiol. 2018;10:61–70. doi: 10.2147/CLEP.S144918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigert J, Steenbergen S. The Connecticut experiment: the role of ultrasound in the screening of women with dense breasts. Breast J. 2012;18:517–522. doi: 10.1111/tbj.12003. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Breast Imaging. Mainiero MB, Moy L, Baron P, Didwania AD, diFlorio RM, Green ED, et al. ACR Appropriateness Criteria® breast cancer screening. J Am Coll Radiol. 2017;14:S383–S390. doi: 10.1016/j.jacr.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Lee EH, Park B, Kim NS, Seo HJ, Ko KL, Min JW, et al. The Korean guideline for breast cancer screening. J Korean Med Assoc. 2015;58:408–419. [Google Scholar]
- 22.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al. J-START investigator groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan strategic anti-cancer randomized trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 23.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20:734–742. doi: 10.1007/s00330-009-1588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brem RF, Tabár L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274:663–673. doi: 10.1148/radiol.14132832. [DOI] [PubMed] [Google Scholar]
- 26.Wilczek B, Wilczek HE, Rasouliyan L, Leifland K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol. 2016;85:1554–1563. doi: 10.1016/j.ejrad.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin Imaging. 2013;37:480–486. doi: 10.1016/j.clinimag.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Japanese Association of Breast and Thyroid Sonology. Guidelines for breast ultrasound diagnosis. 3rd ed. Tokyo: Nankodo; 2014. pp. 111–123. [Google Scholar]
- 29.Ban K, Tsunoda H, Suzuki S, Takaki R, Sasaki K, Nakagawa M. Verification of recall criteria for masses detected on ultrasound breast cancer screening. J Med Ultrason (2001) 2018;45:65–73. doi: 10.1007/s10396-017-0778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology. 2013;269:701–712. doi: 10.1148/radiol.13122829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg WA, Zhang Z, Cormack JB, Mendelson EB. Multiple bilateral circumscribed masses at screening breast US: consider annual follow-up. Radiology. 2013;268:673–683. doi: 10.1148/radiol.13122251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gazhonova V. 3D automated breast volume sonography: a practical guide. Berlin: Springer; 2015. pp. 11–12. [Google Scholar]
- 33.Chae EY, Shin HJ, Kim HJ, Yoo H, Baek S, Cha JH, et al. Diagnostic performance of automated breast ultrasound as a replacement for a hand-held second-look ultrasound for breast lesions detected initially on magnetic resonance imaging. Ultrasound Med Biol. 2013;39:2246–2254. doi: 10.1016/j.ultrasmedbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Jiang YX, Zhu QL, Zhang J, Dai Q, Liu H, et al. Accuracy of an automated breast volume ultrasound system for assessment of the pre-operative extent of pure ductal carcinoma in situ: comparison with a conventional handheld ultrasound examination. Ultrasound Med Biol. 2013;39:2255–2263. doi: 10.1016/j.ultrasmedbio.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 35.van Zelst JCM, Mann RM. Automated three-dimensional breast US for screening: technique, artifacts, and lesion characterization. Radiographics. 2018;38:663–683. doi: 10.1148/rg.2018170162. [DOI] [PubMed] [Google Scholar]
- 36.Skaane P, Gullien R, Eben EB, Sandhaug M, Schulz-Wendtland R, Stoeblen F. Interpretation of automated breast ultrasound (ABUS) with and without knowledge of mammography: a reader performance study. Acta Radiol. 2015;56:404–412. doi: 10.1177/0284185114528835. [DOI] [PubMed] [Google Scholar]
- 37.Chae EY, Cha JH, Kim HH, Shin HJ. Comparison of lesion detection in the transverse and coronal views on automated breast sonography. J Ultrasound Med. 2015;34:125–135. doi: 10.7863/ultra.34.1.125. [DOI] [PubMed] [Google Scholar]
- 38.Vourtsis A, Kachulis A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women. Eur Radiol. 2018;28:592–601. doi: 10.1007/s00330-017-5011-9. [DOI] [PubMed] [Google Scholar]
- 39.Zheng FY, Yan LX, Huang BJ, Xia HS, Wang X, Lu Q, et al. Comparison of retraction phenomenon and BI-RADS-US descriptors in differentiating benign and malignant breast masses using an automated breast volume scanner. Eur J Radiol. 2015;84:2123–2129. doi: 10.1016/j.ejrad.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Lai XJ, Zhu QL, Wang HY, Jiang YX, Liu H, et al. Interobserver agreement for sonograms of breast lesions obtained by an automated breast volume scanner. Eur J Radiol. 2012;81:2179–2183. doi: 10.1016/j.ejrad.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 41.Grubstein A, Rapson Y, Gadiel I, Cohen M. Analysis of false-negative readings of automated breast ultrasound studies. J Clin Ultrasound. 2017;45:245–251. doi: 10.1002/jcu.22474. [DOI] [PubMed] [Google Scholar]
- 42.van Zelst JCM, Tan T, Platel B, de Jong M, Steenbakkers A, Mourits M, et al. Improved cancer detection in automated breast ultrasound by radiologists using computer aided detection. Eur J Radiol. 2017;89:54–59. doi: 10.1016/j.ejrad.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Inciardi MF, Edwards AV, Papaioannou J. Interpretation time using a concurrent-read computer-aided detection system for automated breast ultrasound in breast cancer screening of women with dense breast tissue. AJR Am J Roentgenol. 2018;211:452–461. doi: 10.2214/AJR.18.19516. [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Bao L, Tan Y, Zhu L, Kong F, Wang W. 1000-case reader study of radiologists' performance in interpretation of automated breast volume scanner images with a computer-aided detection system. Ultrasound Med Biol. 2018;44:1694–1702. doi: 10.1016/j.ultrasmedbio.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 45.van Zelst JCM, Tan T, Clauser P, Domingo A, Dorrius MD, Drieling D, et al. Dedicated computer-aided detection software for automated 3D breast ultrasound; an efficient tool for the radiologist in supplemental screening of women with dense breasts. Eur Radiol. 2018;28:2996–3006. doi: 10.1007/s00330-017-5280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weigert JM. The Connecticut experiment; the third installment: 4 years of screening women with dense breasts with bilateral ultrasound. Breast J. 2017;23:34–39. doi: 10.1111/tbj.12678. [DOI] [PubMed] [Google Scholar]




