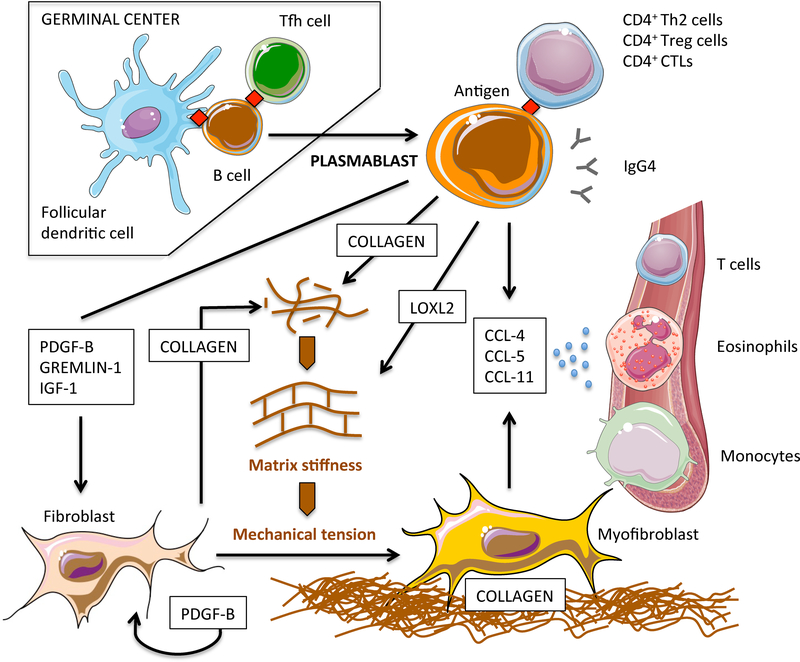
Figure 6. Revised pathogenetic model of IgG4-RD pathogenesis: B-cell contribution to tissue fibrosis.
After germinal centre reaction, antigen experienced naïve B cells differentiate into class-switched plasmablasts and migrate into inflamed sites where they contribute to tissue fibrosis in different ways. Plasmablasts initiate fibroblast differentiation into myofibroblasts through soluble factors that activate transcriptional programs implicated in mesenchymal transition. Plasmablasts also stimulate fibroblasts to secrete collagen and PDGF-B, thus amplifying fibroblast activation in an autocrine/paracrine loop. Plasmablasts directly contribute to tissue fibrosis by secreting collagenous proteins. Plasmablasts regulate extracellular matrix stiffness by secreting enzymes responsible for the crosslinking of collagen molecules such as LOXL2. Increased extracellular matrix stiffness leads to the activation of mechanoceptors on fibroblasts, thus fostering their full differentiation into myofibroblasts. Plasmablasts attract inflammatory cells with additional fibrotic potential such as CD4+ CTLs, eosinophils, and M2 macrophages, by secreting CCL-4, CCL-5, and CCL-11, and by stimulating fibroblasts to produce these same chemokines. The pathogenic relevance of IgG4 antibodies and of T-cell populations presumably sustained by cognate antigen specific plasmablasts remains to be defined.