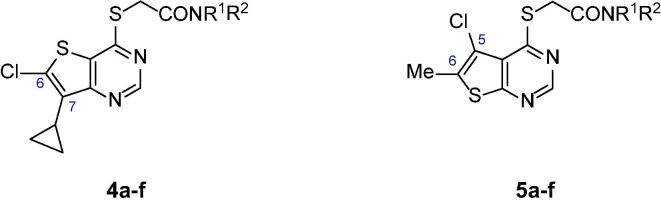Table 1.
| NR1R2 | Compound | Notumb IC50 (nM) |
MLMc Cli (μL/min/mg) |
MDCK-MDR1c AB/BA Papp (×10−6 cm/s) and efflux ratio (ER) |
|
|---|---|---|---|---|---|
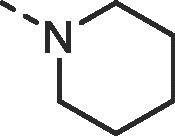
| –NMe2 | 4a | 7.5 ± 12.4 | |||
| 5a | 15 ± 6 | 360 | |||
 |
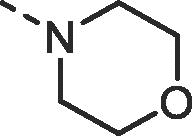
4b | 91 ± 67 | |||
| 5b | 220 ± 12 | ||||
 |
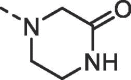
4c | 18 ± 8.7 | >500 | 40/38 | 0.95 |
| 5c | 69 ± 10 | ||||
 |
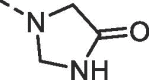
4d | 7.1 ± 4.1 | 24 | 7.9/65 | 8.2 |
| 5d | 5.8 ± 4.0 | 19 | 12/66 | 5.5 | |
 |
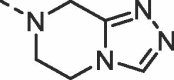
4e | 1.5 ± 0.1 | 19 | 3.8/14 | 3.7 |
| 5e | 2.7 ± 0.5 | 65 | 14/82 | 5.9 | |
 |
4f | 1.1 ± 0.3 | 29 | 0.95/93 | 98 |
| 5f | 3.2 ± 0.1 | 13 | 0.6/56 | 93 | |
See Ref. 12.
All values are geometric mean ± s.d. of n = 2–6 experiments quoted to 2 s.f. Differences of <2-fold should not be considered significant. For details of the assay protocol, see reference 15.
MLM, MDCK-MDR1 and additional in vitro ADME studies reported in this work were independently performed by GVK Biosciences (Hyderabad, India. https://www.gvkbio.com/discovery-services/biology-services/dmpk-services/) or Cyprotex (Macclesfield, UK. https://www.cyprotex.com/admepk).