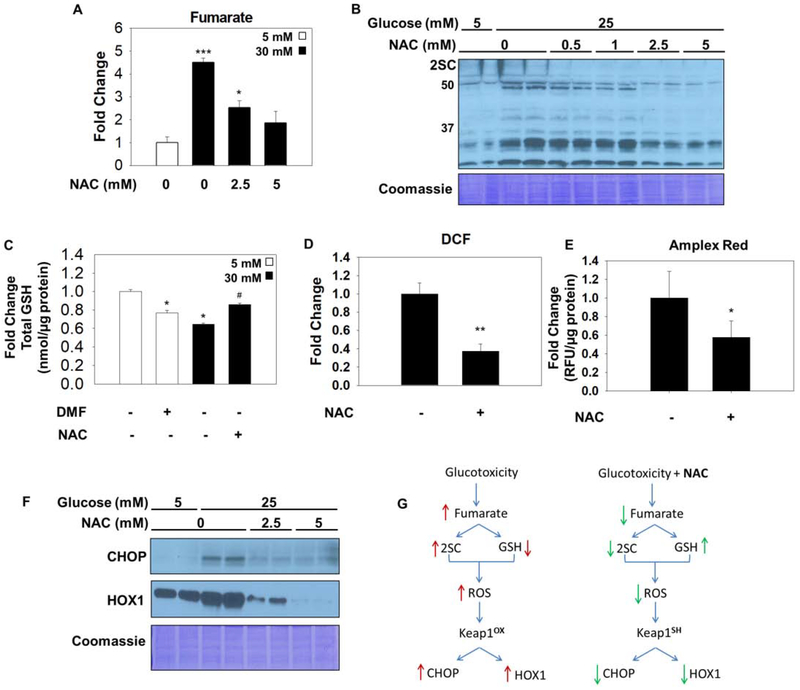
Figure 4: N-acetylcysteine reduces fumarate, protein succination and ROS.
(A) Metabolites were extracted from adipocytes matured in 5 or 25 mM glucose, or 25 mM glucose treated with 5 mM NAC for 3 days. Fumarate levels are significantly increased with glucotoxicity and return to normal with NAC treatment. Data is normalized to total protein content (μg) (n=3/group, mean +/− SEM, *p< 0.05, ***p<0.001 vs. 5 mM glucose, ##p<0.01 vs. 30 mM glucose). (B) 30 μg of protein from adipocytes cultured in 5 or 25 mM glucose and treated with 0, 0.5, 1, 2.5 or 5 mM N-acetylcysteine (NAC) for 8 days was immunoblotted to detect 2SC levels. (C) (D) Adipocytes were treated with 100 μM dimethyl fumarate (DMF) for 24 hours, or 5 mM NAC for 8 days. Total glutathione levels were quantified (n=3/group, mean +/− SEM, *p< 0.05 vs. 5 mM glucose, #p<0.05 vs. 30 mM glucose). Reactive oxygen species was measured using dichlorofluorescein in adipocytes matured in 30 mM glucose with or without 1 mM NAC for 8 hours (n=4/group, mean +/− SEM, **p<0.01). (E) CHOP and HOX1 levels were measured in adipocytes treated with 2.5 or 5 mM NAC for 8 days. Coomassie staining represents equal protein loading. (F) Schematic summarizing beneficial effect of N-acetylcysteine during glucotoxicity. Elevated glucose increases fumarate levels resulting in protein succination, reduced glutathione (GSH) concentrations, and exacerbates the levels of reactive oxygen species (ROS) that react with redox-sensitive cysteines on Keap1. Oxidized Keap1 is unable unable to form the CSN supercomplex or to sequester Nrf2, resulting in the accumulation of CHOP and increased production HOX1. During high glucose stress N-acetylcysteine (NAC) decreases fumarate concentrations and protein succination and rescues the concentration of GSH in the adipocyte resulting in decreased ROS levels. In the absence of oxidative stress Keap1 is able to promote CHOP degradation and down regulate HOX1 production by sequestering Nrf2 in the cytosol.