Short abstract
Aims
New therapeutics for the control of influenza virus infections are needed to alleviate the burden caused by seasonal epidemics and occasional pandemics, and to overcome the potential risk of drug-resistance emergence. Enisamium iodide (Amizon®, Farmak) is currently approved for clinical use for the treatment of influenza in 11 countries which includes Ukraine, Russia, Belarus, Kazakhstan, and Uzbekistan. However, experimental evidence of the antiviral activity of enisamium has not been reported.
Methods
Antiviral activity of enisamium was assessed by virus yield reduction assays using differentiated normal human bronchial epithelial cells. Permeability of enisamium into differentiated normal human bronchial epithelial cells and its cytotoxicity were also assessed, and comparisons with other cell lines were made.
Results
Enisamium inhibited replication of multiple subtypes of influenza A viruses, including seasonal H1N1, 2009 pandemic H1N1, seasonal H3N2, the zoonotic H5N1 and H7N9, neuraminidase inhibitor-resistant variant carrying the H275Y NA substitution (N1 numbering), and influenza B virus at doses 23- to 64-fold lower than cytotoxic concentrations. The permeability of enisamium in Madin–Darby canine kidney cells (where no antiviral activity was found) was less than 0.08%, while higher permeability was observed in differentiated normal human bronchial epithelial cells (1.9%). The kinetics of enisamium intracellular uptake in differentiated normal human bronchial epithelial cells was concentration dependent. In time-of-addition experiments in differentiated normal human bronchial epithelial cells, enisamium treatment within 4 h after A(H1N1) virus inoculation resulted in 100-fold or greater reductions in virus titers, suggesting that it affects an early stage of the virus life cycle.
Conclusions
Enisamium exhibits antiviral activity against influenza viruses in vitro, supporting the reported clinical efficacy against influenza virus infections.
Keywords: Influenza virus, antiviral, normal human bronchial epithelial cells, enisamium
Introduction
Influenza is an acute respiratory illness caused by influenza A and B viruses resulting in annual epidemics, and occasional pandemics with significant morbidity and mortality. Although vaccines have been proven effective in mitigating the illness and the severity of influenza infections, the strain composition of the seasonal vaccine requires annual evaluation and reformulation. Due to the considerable time required to develop vaccines, the intended efficacy may not be achieved following immunization because of the emergence of new antigenic variants caused by antigenic drift or strain mismatch. Therefore, effective antiviral therapies are essential for immediate intervention against influenza infections. Currently, two members of a single class of viral neuraminidase (NA) inhibitors, oseltamivir and zanamivir, are recommended for prophylaxis and treatment of influenza in adults and children worldwide.1 Emergence of NA inhibitor-resistant influenza viruses is a major public health concern. Between 2007 and 2009, the high prevalence (∼97%) of oseltamivir-resistant seasonal influenza A(H1N1) viruses carrying the H275Y NA substitution (N1 numbering) was reported globally.2,3 Although the detection rate of NA inhibitor resistance was initially low (∼1% of A(H1N1)pdm09 viruses are resistant to oseltamivir),4 reports of increases in the prevalence of A(H1N1)pdm09 influenza viruses with the H275Y NA substitution (from >1.0 to 2.5%) in Japan in 20135,6 and isolation of community-transmitted, oseltamivir-resistant viruses7,8 suggest that this substitution is not deleterious to the fitness of the virus. Thus, these oseltamivir-resistant viruses have the potential to spread among humans. For this reason, there is an urgent need for novel antiviral agents that can effectively control influenza.
Currently, extensive efforts are being undertaken to identify novel drug targets, including both virus protein- and host-targeted compounds, and to further develop these compounds as antivirals for treating influenza virus infections. Here, we studied the antiviral potential of an isonicotinic acid derivative, enisamium, against influenza A and B viruses in vitro. Enisamium iodide is the active compound of Amizon® (Figure 1), which is licensed and marketed in 11 countries, including Ukraine, Russia, Belarus, Kazakhstan, Uzbekistan, as an antiviral agent against influenza.9 The effectiveness of Amizon® in treating patients with uncomplicated influenza was demonstrated in the phase 3 clinical study conducted in Russia.10 However, experimental evidence for the efficacy of enisamium against influenza virus infection was not reported until recently.11 In that brief communication, enisamium was shown to inhibit an influenza A (H1N1) virus infection in vitro and also to significantly reduce influenza A (H3N2) viral titers in infected ferrets. This report is an extension of the prior published work where we demonstrate that enisamium exhibits antiviral activity against different types of influenza virus in cell culture. Mode of action experiments show that viral RNA synthesis and subsequent viral protein expression are inhibited by treatment of infected cells with the drug, although the manner in which this occurs was not elucidated. These studies support the reported clinical efficacy of enisamium against influenza virus infections.
Figure 1.
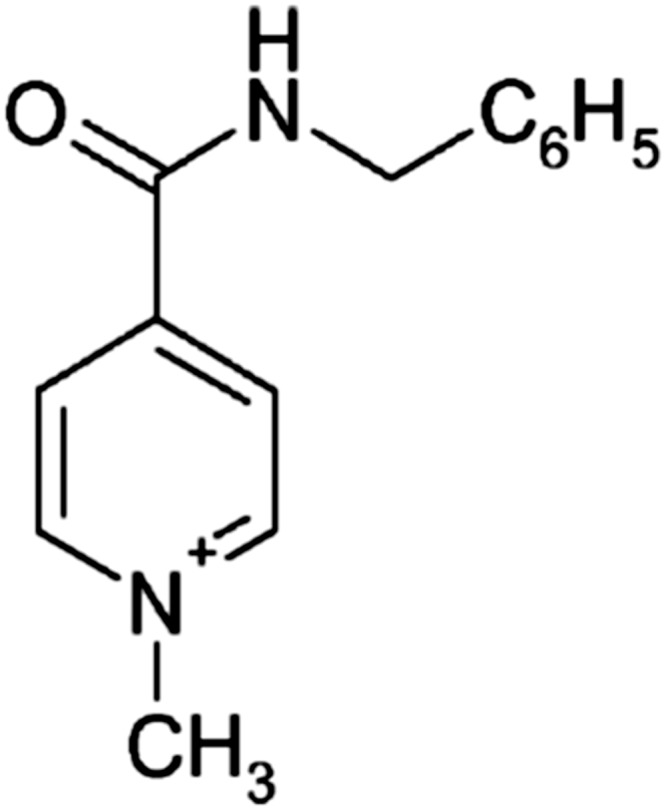
Chemical structures of enisamium (N-methyl-4-benzylcarbamidopyridinium).
Materials and methods
Antiviral compounds
Iodide and chloride salt forms of enisamium (Figure 1) were provided by Farmak (Kiev, Ukraine). These were prepared as 160 mM stocks in sterile distilled water. The viral NA inhibitor oseltamivir carboxylate was purchased from Toronto Research Chemicals (Toronto, Canada) and was prepared as 5 mM stock in sterile distilled water.
Cells
Madin–Darby canine kidney (MDCK) cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in modified Eagle’s medium (MEM; Cellgro, Manassas, VA), supplemented with 5% fetal calf serum (HyClone, Logan, UT). Undifferentiated and differentiated normal human bronchial epithelial (dNHBE) cells were purchased from MatTek’s EpiAirway System (MatTek, Ashland, MA) and maintained in Opti-MEM (Fisher, Grand Island, NY), supplemented with 10% fetal bovine serum (HyClone, Logan, UT). The cells were obtained from a single donor (donor #9831) and were used throughout the experiments for assay consistency. dNHBE cells were grown in culture in an air–liquid interface system on transwell inserts (Corning, Tewksbury, MA). The apical surface of the cells was exposed to a humidified 95% air and 5% CO2 environment. The basolateral medium was changed and the mucin layer was washed with sterile phosphate buffered saline every 24–48 h.
Influenza viruses
Influenza A/Georgia/20/2006 (H1N1) H275Y (oseltamivir-resistant), A/Brisbane/59/2007 (H1N1), A/Tennessee/1–560/2009 (H1N1)pdm09, A/Perth/16/2009 (H3N2), A/Vietnam/1203/2004 (H5N1), A/Anhui/1/2003 (H7N9), and B/Texas/06/2011 viruses were obtained from the Influenza Division at the Centers for Disease Control and Prevention, and St Jude Children’s Research Hospital. Viruses were propagated in MDCK cells for 48 h at 33–37°C in serum-free MEM containing 1 µg/ml of l-tosylamido 2-phenylethyl chloromethyl ketone-treated trypsin (Worthington, Lakewood, NJ).
Cytotoxicity assay
The viability of dNHBE cells was tested 24 h after incubation with enisamium by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Sigma-Aldrich, St Louis, MO) according to the manufacturer’s instructions. The mean value of the negative control in each plate was set at 100%, and the percentage of absorbance of the compound-containing well was determined in relation to this internal control. The 50% cellular cytotoxicity (CC50) was determined by using the four-parameter logistic nonlinear regression model equation in GraphPad Prism 5 software (GraphPad Software, La Jolla, CA).
Virus yield reduction assay
Enisamium was added to the basal compartment of dNHBE cells 24 h prior to influenza virus inoculation. Oseltamivir carboxylate (1 µM) was added to the basal compartment of dNHBE cells 1 h prior to influenza virus inoculation and maintained in this compartment. Influenza A or B viruses (MOI of 0.01 PFU/cell) were added to the apical compartment of the transwell inserts. After 1 h adsorption, enisamium (40, 200, 600, or 1000 µM for seasonal viruses or at 100, 500, and 1000 µM for A/Vietnam/1203/2004 (H5N1) and A/Anhui/1/2013 (H7N9) viruses) was added to the basal compartment and incubated for 48 h at 37°C under 5% CO2. Following virus inoculation and drug treatment, dNHBE cells were maintained at the air–liquid interface, and viruses released into the apical compartment of dNHBE cells were harvested at 48 h by washing the cells with 0.5 ml of serum-free bronchial epithelial cell growth medium (MatTek, Ashland, MA). The virus-containing apical washes were stored at −70°C until use. The 50% tissue culture infectious doses (TCID50s) were determined in MDCK cells after inoculation with 10-fold dilutions of the apical washes and incubation at 37°C in 5% CO2 for 48–72 h. The TCID50 titers were calculated by the method of Reed and Muench.12
Intracellular uptake and extracellular release of enisamium
MDCK, undifferentiated NHBE and dNHBE cells were overlaid with prewarmed, serum-free medium containing enisamium (100 µM of enisamium iodide for MDCK cells and 2000 µM of enisamium chloride for undifferentiated and dNHBE cells), and incubated at 37°C under 5% CO2 for 24 h (n = 3 wells). At 0.25, 0.5, 1, 2, 4, 8, and 24 h after removal (release) or after addition of enisamium (uptake), the supernatant (media) was removed from the individual wells, and the cells were immediately washed once with 1 ml of saline solution (0.9% sodium chloride injection USP, Hospira, Lake Forest, IL). The cells were lysed with the addition of methanol (100%), and the cell lysate was analyzed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) and methods established at IITRI to determine the uptake of enisamium.
Time-of-addition experiment
dNHBE cells were inoculated with influenza A/Brisbane/59/2007 (H1N1) virus at an MOI of 1.0 PFU/cell (time zero) and a single dose of enisamium (2000 µM) was added to the basal compartment at −1, 0, 2, 4, 6, 8, or 10 h postinoculation (hpi). Cultures were incubated at 37°C, and culture supernatants were collected at 24 hpi for virus yield determination in MDCK cells by TCID50 assay.
RNA isolation and quantitation
At the termination of certain experiments, 1 ml Trizol (Invitrogen) was added to each well for RNA isolation. Total RNA was isolated from cells using Trizol following the manufacturer’s instruction. Total RNA was dissolved in RNase-free water and quantitated using Biomate 5 spectrophotometer (Thermo Electron Corp., Madison, WI) at 260/280. When extra RNA was available, RNA samples were subjected to electrophoresis analysis to ensure RNA was not degraded.
qRT PCR: RT-PCR
qRT PCR: RT-PCR was performed using a standard procedure. Briefly, the reverse transcriptase (RT) assay was done in a final volume of 20 µl with 2 µg total RNA and 80 units of MuLV RT (Invitrogen) at 42°C for 50 min. Duplicate RT samples (cDNA) for each RNA sample were generated, then the two cDNA samples were pooled and diluted with RNAse-free water by twofold, so in total 80 µl cDNA for each sample was generated and stored at −80°C until further qPCR analysis. Real-time PCR was performed with 2 µl diluted cDNA in a MyiQ Real-time PCR Detection System (Bio-Rad, Hercules, CA) using iQTM SYBR Green PCR Supermix (Bio-Rad) according to the Manufacturer guidelines.
The PCR cycling conditions used were as follows: 40 cycles of 15 s at 95°C, 15 s at 60°C, and 20 s at 72°C. Fold inductions were calculated using the formula 2−(ΔΔCt), where ΔΔCt is ΔCt(treatment) − ΔCt(control), ΔCt is Ct(target gene) – Ct(gapdh) and Ct is the cycle at which the threshold is crossed. The gene-specific primer pairs (and product size) for the real-time PCR were designed using primer 3 program (http://bioinfo.ut.ee/primer3-0.4.0/). GAPDH served as a reference gene for normalization.
Western blot analysis
At each time point of sample collection, cells were washed and RIPA lysis buffer (containing protease inhibitors) (150 µl) was added to each well to lyse the cells. Lysed cells were collected into a 1.5 ml tube, centrifuged at 14,000 g × 5 min and the supernatant was transferred to new tubes (50 µl/tube) and stored at −65°C until analysis. The protein concentration of the supernatant was determined by the Bradford method. Thirty micrograms of the protein extracts was electrophoresed on a 10% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membrane. The membrane was blocked with 5% nonfat dry milk in TBS for 1 h and then incubated at room temperature for 1.5 h with corresponding antibodies that were diluted 1:1000 with blocking buffer. The membrane was washed 3 × 10 min in TBST (0.1% Tween in TBS) and then incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit/anti-mouse secondary antibody (Santa Cruz Biotechnology, Dallas, TX), both of which were diluted 1:2500 with blocking buffer. After incubation with secondary antibody, the membrane was washed 3 × 10 min with TBST and subjected to ECL detection with an ECL kit (Santa Cruz Biotechnology). Specific polyclonal antibody against HA (A/Brisbane/59/2007) was purchased from MyBioSource, Inc. (MBS42100, San Diego, CA). Specific mouse monoclonal antibody against HA (A/Brisbane/59/2007) was purchased from eEnzyme, LLC (MIA0019, Gaithersburg, MD). β-actin served as a loading control and was detected on the same membrane. HRP-labeled β-actin antibody (mAb) was from GenScript (A00730-40, Piscataway, NJ).
Data analysis
Normally distributed parametric data are presented as mean ± SD and were analyzed by Student’s t-test. Statistical analyses were performed using GraphPad Software (San Diego, CA) or Microsoft Office Excel. Differences between means were considered to be significant at P < 0.05.
Results
Antiviral activity and cytotoxicity of enisamium
To evaluate the antiviral efficacy of enisamium against influenza viruses in vitro, we initially performed assays in MDCK cells, the most widely used cell line for replication of influenza viruses and for antiviral compounds testing.13,14 Incubation of MDCK cells with enisamium prior to and during influenza A/Brisbane/59/2007 (H1N1) virus inoculation did not reduce plaque number/size or virus titers in the plaque and virus yield reduction assays, respectively (data not shown). Therefore, we searched for other cell systems that demonstrated productive influenza virus infection to determine the antiviral activity of enisamium. dNHBE cells cultured at the air–liquid interface form a multilayered differentiated model which closely resembles the epithelial tissue of the human respiratory tract. dNHBE cells grown in this manner retain physiological functions and morphological attributes of the upper respiratory tract and are known to permit influenza virus infection.15 Fortuitously, we discovered that enisamium inhibited influenza A and B viruses in these cells. Therefore, all subsequent examinations of the antiviral activity of enisamium against influenza viruses were conducted in dNHBE cells using optimized conditions but required using a virus yield reduction assay. This is because no viral cytopathic effect was observed when these cells were infected with influenza viruses. Overall, enisamium inhibited the replication of influenza viruses with a 90% effective (EC90) concentration range of 157–439 µM. It appeared that the influenza B virus was slightly more inhibited by enisamium than were the influenza A viruses (Table 1).
Table 1.
Antiviral activity of enisamium against seasonal influenza A and B viruses and viruses with pandemic potential in differentiated NHBE cells.
| Influenza A and B virus | EC90 (µM)a | Selectivityindexb |
|---|---|---|
| Seasonal influenza virus | ||
| A/Georgia/20/2006 (H1N1) H275Yc | 144; 286 | 46 |
| A/Brisbane/59/2007 (H1N1) | 412; 466 | 23 |
| A/Tennessee/1-560/2009 (H1N1)pdm09 | 233; 351 | 34 |
| A/Perth/16/2009 (H3N2) | 287; 375 | 30 |
| B/Texas/06/2011 | 105; 209 | 64 |
| Pandemic potential influenza virus | ||
| A/Vietnam/1203/2004 (H5N1) | 400 | 25 |
| A/Anhui/1/2013 (H7N9) | 410 | 24 |
aData represent 90% reduction (1 log10TCID50/ml) in virus yield. Actual values are given for two independent assays, except for single assays performed for H5N1 and H7N9 viruses. Virus yield was determined by titration in MDCK cells, and the data represent titer reductions compared to untreated cells. Oseltamivir carboxylate was used as a positive control at 1 µM, and at that concentration it inhibited all viruses (EC90 <1 µM) except for the A/Georgia/20/2006 (H1N1) virus with H275Y NA substitution (no inhibition at 1 µM).
bSelectivity index (or SI value, which is CC50 divided by EC90) based on cytotoxicity determination is performed in parallel in dNHBE cells. The mean 50% cytotoxic concentration was 9980 (8660–11,300) µM.
cA/Georgia/20/2006 virus carries H275Y NA substitution (N1 numbering) and is resistant to oseltamivir carboxylate.
A critical requirement of novel therapeutic compounds is the lack of the adverse effects on host cell processes that may lead to cell death. Therefore, we determined the cytotoxicity of enisamium in dNHBE cells in parallel with the antiviral assays. The 50% cytotoxic concentration (CC50) in serum-free media was just under 10 mM (Table 1, footnote “c”). Thus, the selectivity index values (CC50 divided by EC90) for enisamium against the viruses ranged from 23 to 64 (Table 1). This indicates that enisamium is not cytotoxic at antiviral concentrations used.
Intracellular uptake of enisamium
To understand why antiviral activity of enisamium is observed in dNHBE cells but not in MDCK cells, we determined whether differences in intracellular uptake of enisamium in MDCK, undifferentiated NHBE and dNHBE cells (Table 2) could be involved. Permeability of enisamium was determined in these three cell systems, and the highest uptake (1.9%) was observed in dNHBE cells. To determine whether increasing compound concentrations affected intracellular concentrations and permeability of enisamium, dNHBE cells were incubated with varying compound concentrations. After 24 h of exposure with 10, 50, 100, 500, and 1000 µM of enisamium, the intracellular drug concentrations were 36.8, 213, 410, 1727, and 3009 ng/106 cells, respectively (Table 3). These results indicated that the uptake of enisamium into the dNHBE cells is dependent on extracellular drug concentration, albeit the permeability of enisamium remained low (1.1–1.6%) for all concentrations tested. To establish how intracellular uptake of enisamium increases over time, dNHBE cells were incubated with enisamium (1000 µM) for different periods of time. Exposure to enisamium for 0.25–24 h resulted in 482–2732 ng/106 cells of enisamium uptake, respectively (Figure 2(a)). Thus, the intracellular concentration of enisamium increased over time through 24 h.
Table 2.
Intracellular uptake of enisamium in different host cell systems.
| Cell system | Concentration of enisamium (mean ± SD, ng/106 cells)a |
Permeability(mean ± SD, %)b | |
|---|---|---|---|
| Initial input | Intracellular | ||
| MDCK cells | 35,300 ± 1700 | 27.9 ± 1.4 | 0.08 ± 0.01 |
| Undifferentiated NHBE cells | 487,000 ± 2200 | 4110 ± 830 | 0.85 ± 0.21 |
| Differentiated NHBE cells | 568,000 ± 42,000 | 10,700 ± 1900 | 1.90 ± 0.20 |
MDCK: Madin–Darby canine kidney; NHBE: normal human bronchial epithelial.
aThe cells were overlaid with medium containing enisamium (100 µM of enisamium iodide for MDCK cells and 2000 µM of enisamium chloride for undifferentiated and dNHBE cells) and incubated at 37°C under 5% CO2 for 24 h (n = 3 wells). The cellular lysates and supernatants were harvested and the concentration of enisamium was determined using LC–MS/MS. Concentration is expressed as nanograms (ng) per 106 cells.
bPermeability of enisamium was calculated as the ratio of the intracellular concentration to that in the initial drug input, and expressed as percentage.
Table 3.
Permeability of different concentrations of enisamium in differentiated NHBE cells.
| Enisamium (µM) | Intracellular concentration of enisamium (mean ± SD, ng)a | Permeability (mean ± SD, %)b |
|---|---|---|
| 10 | 36.8 ± 3.9 | 1.40 ± 0.15 |
| 50 | 213 ± 34 | 1.62 ± 0.26 |
| 100 | 410 ± 77 | 1.56 ± 0.29 |
| 500 | 1727 ± 108 | 1.31 ± 0.08 |
| 1000 | 3009 ± 132 | 1.15 ± 0.05 |
NHBE: normal human bronchial epithelial.
aThe serum-free bronchial epithelial cell growth medium containing enisamium at the indicated concentration was added to the basal compartment of differentiated NHBE cells and incubated at 37°C under 5% CO2 for 24 h (n = 3 wells/concentration). The cellular extracts were harvested and the concentration of enisamium was determined using LC–MS/MS. Concentration is expressed as nanograms (ng) per 106 cells.
bPermeability was calculated as the ratio of the intracellular concentration to that in the initial input (converted to µM using the molecular weight of enisamium chloride salt of 262.737 g/mol).
Figure 2.
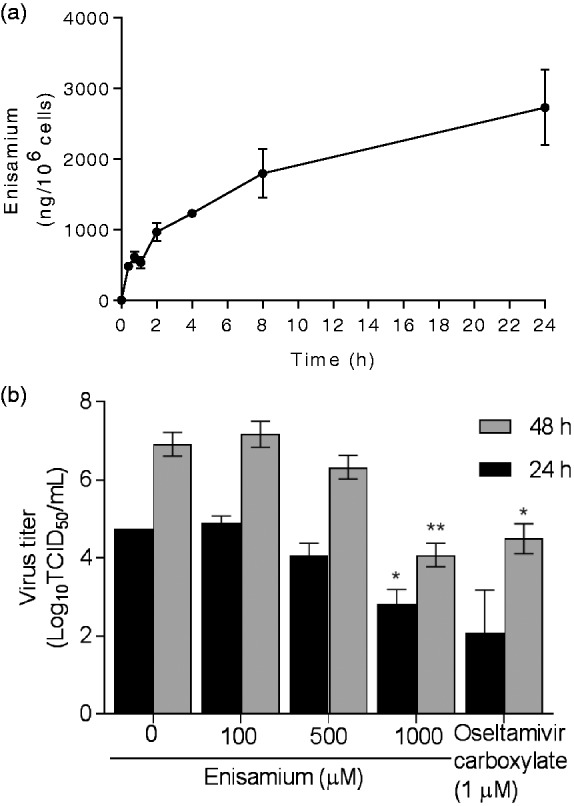
Kinetics of intracellular uptake (a) and effect of different concentrations and durations of enisamium treatment on dNHBE cells prior to influenza A/Brisbane/59/2007 (H1N1) virus inoculation (b). (a) Enisamium (2000 µM) was added to the basal compartment of uninfected dNHBE cells. The cell lysates were collected at 0.25, 0.5, 1, 2, 4, 8, and 24 h after exposure for determination of intracellular drug concentrations. (b) dNHBE cells were pretreated with enisamium (100, 500, or 1000 µM) 24 h prior to inoculation with influenza A/Brisbane/59/2007 (H1N1) virus (MOI of 0.001 PFU/cell). Enisamium was maintained in the basal compartment throughout the experiment. Oseltamivir carboxylate (final concentration, 1 µM) was added to the basal compartment 1 h before virus inoculation and remained for 24 or 48 h. The time of viral inoculation is indicated as 0 h. Supernatants were collected 24 (black bars) and 48 (gray bars) hpi, and virus titers were determined in MDCK cells by the TCID50 assay at 37°C and expressed as log10TCID50/ml. Statistical significance was tested by comparing to virus-inoculated untreated NHBE cells by unpaired Student's t-test (*denotes P≤0.05 and **P ≤ 0.01 (as determined by unpaired t-test). TCID50: 50% tissue culture infectious dose.
Effect of exposure time on the antiviral activity of enisamium
To determine whether preexposure of dNHBE cells with enisamium may enhance the antiviral effect, enisamium was added to cells 24 h prior to infection with influenza A/Brisbane/59/2007 (H1N1) virus. Enisamium remained on infected cells for an additional 24 or 48 h. At the time of virus inoculation (exposure to enisamium for 24 h), incubation with 100, 500, and 1000 µM of enisamium resulted in intracellular uptake of 525, 1885, and 3488 ng/106 cells, respectively (which correlates with the data in Table 3). These levels increased to 719, 2860, and 4805 ng/106 cells by 48 hpi. Enisamium inhibited virus replication at 24 and 48 h postinfection at the 1000 µM concentration, as did oseltamivir carboxylate (1 µM). Incubation of dNHBE cells with enisamium did not appreciably show an antiviral effect when drug was used at the lower concentrations (100 and 500 µM). At the highest concentration (1000 µM) of enisamium, the viral titers were reduced by 1.9 and 2.8 log10TCID50/ml at 24 and 48 hpi, respectively (Figure 2(b)). These data suggest that the lack of antiviral activity of enisamium at a lower concentration was due to the inability to achieve effective intracellular concentrations. This limitation may be overcome when dNHBE cells are exposed to a higher concentration of enisamium for a prolonged period. Therefore, the intracellular concentration required for achieving an antiviral activity of enisamium exceeds 1885 ng/106 cells.
Time-of-exposure experiment
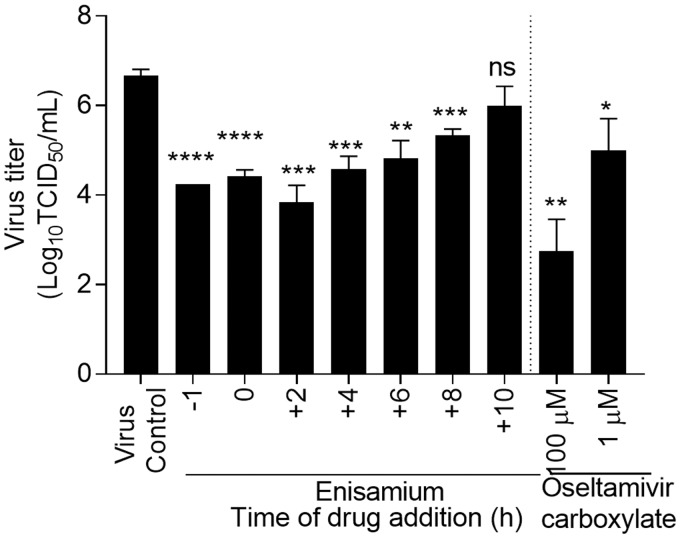
To assess the stage of the influenza viral replication cycle affected by enisamium, a time-of-addition experiment was performed in dNHBE cell infected with A/Brisbane/59/2007 (H1N1) influenza virus (Figure 3). Addition of enisamium 1 h prior to virus inoculation, during virus adsorption, or up to 2–4 hpi resulted in pronounced inhibition of influenza A(H1N1) virus replication of 2.3–2.6 log10TCID50/ml (P < 0.05). These early events encompass virus binding and entry, as well as transcription of viral mRNA and replication of genomic RNA that is dependent on the viral polymerases.16 When compared to earlier time points, enisamium was less effective when added at 8 and 10 hpi, with virus yield reductions of only 1.3 and 0.7 log10TCID50/ml, respectively (Figure 3), and thus is less effective at late stages of replication (predominantly vRNA replication) and/or genome packaging. Reduction of influenza A(H1N1) virus titers by enisamium and oseltamivir carboxylate was dose dependent. Thus, enisamium acts, at least, during the steps in the viral cycle encompassing viral mRNA transcription and genome replication.
Figure 3.
Effect of time-of-enisamium addition on influenza A/Brisbane/59/2007 (H1N1) virus replication in dNHBE cells. Differentiated NHBE cells were inoculated with influenza A/Brisbane/59/2007 (H1N1) virus (MOI of 1.0 PFU/cell) and enisamium (2000 µM) was added to the basal chamber at −1, 0, 2, 4, 6, 8, and 10 h after virus inoculation and remained for 24 h. Oseltamivir carboxylate (final concentration, 1 and 100 µM) was added to the basal chamber 1 h before virus inoculation and remained for 24 h. The time of viral inoculation is indicated as 0 h. Supernatants were collected 24 h p.i. from the upper chamber and stored in aliquots at −80°C until titration on MDCK cells (expressed as log10TCID50/ml). Values are the means ± standard error from triplicate. P ≤ 0.05 as compared to virus titers to virus-inoculated untreated NHBE cells (as determined by unpaired t-test; *≤0.05; **≤0.01; ***≤0.001; ****≤0.0001). TCID50: 50% tissue culture infectious dose.
Effect of enisamium on viral RNA synthesis
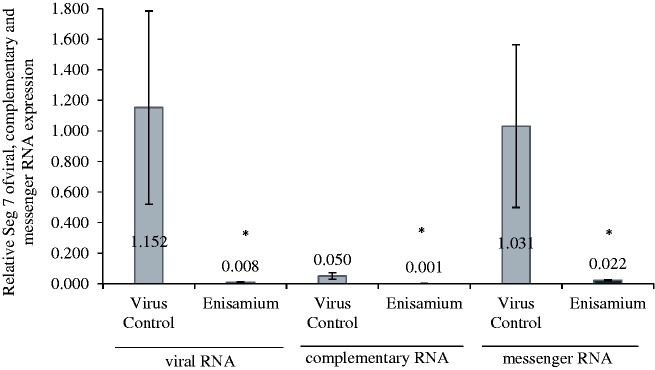
Continuous treatments of infected cells were conducted with enisamium, and RNA samples were taken at varying times postinfection (Figure 4). The samples were analyzed for the presence of the viral M gene, which served as a representative marker of viral RNAs present in the samples. The M gene was not detected at 4 h, but increasing amounts were seen at 8, 12, and 18 h (where it reached its maximum). At each of these time points and at 24 h, viral M gene RNA was lower in enisamium-treated cells versus untreated cells. A second experiment was conducted in which the effects of enisamium treatment on segment 7 viral, complementary, and messenger RNA were determined following a 24 h incubation (Figure 5). Here, there was nearly a complete inhibition of viral RNA synthesis. These results with M gene and segment 7 RNAs demonstrate that enisamium inhibited viral RNA synthesis, although the manner in which this occurred could not be elucidated in the experiments.
Figure 4.
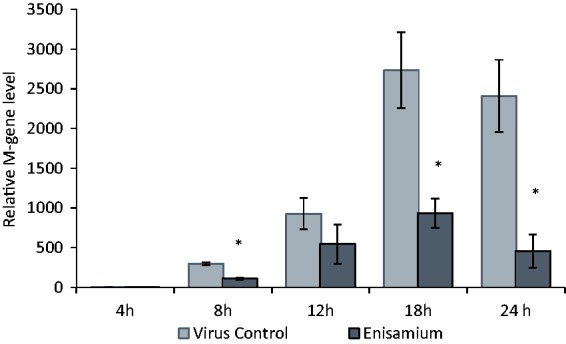
M-gene expression in A/Brisbane/59/2007-infected (MOI 1.0) dNHBE cells after treatment with enisamium (2000 µM) for 4, 8, 12, 18, and 24 h. *p<0.05 in comparison to virus control, n = 3.
Figure 5.
Expression of segment 7 of viral, complementary, and messenger RNA in A/Brisbane/59/2007-infected (MOI 0.01) NHBE cells after treatment with enisamium (1000 µM) for 24 h. *p<0.05 in comparison to virus control, n = 3.
Effect of enisamium treatment on viral protein expression
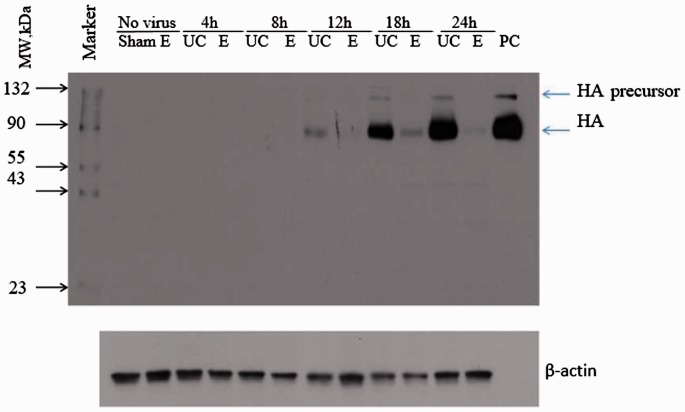
Since viral proteins are derived from viral RNA, it follows that an inhibition of viral RNA (as demonstrated in Figures 4 and 5) by enisamium treatment should lead to a decrease in the expression of viral protein. In order to demonstrate that this occurs, the production of viral hemagglutinin (HA) as a representative viral protein was monitored over a 24 h period by Western blot analysis. HA protein was not detectable or poorly detectable at 4 and 8 h postinfection (Figure 6). The amount of this protein increased over time and was evident at 12, 18, and 24 h. Notably, treatment of cells with enisamium (E in the figure) markedly decreased the amount of detectable HA protein, confirming that the decrease in viral RNA synthesis led to a decrease in viral protein synthesis.
Figure 6.
Effect of enisamium (E) treatment compared to untreated control (UC) on HA protein expression in dNHBE cells (MOI: 1.0; enisamium: 2000 µM; treatment: 4 to 24 h). Western blot analysis of HA protein expression. NHBE cells were infected with A/Brisbane/59/2007 (MOI 1.0) at T0, cells were treated from −1 to 4, −1 to 8, −1 to 12, −1 to 18, and −1 to 24 h. Samples were collected after each treatment period and subjected to Western blot analysis with A/Brisbane/59/2007 HA specific polyclonal antibody. Exposure time: 1 min; PC: positive control (HA protein control); HA: hemagglutinin.
Discussion
The NA inhibitors are the only virus protein-specific antivirals currently available for control of influenza virus infections worldwide.17 Overreliance upon NA inhibitors increases the risk of antiviral resistance and justifies the pursuit of novel inhibitors that target different viral and/or host targets. Here, we demonstrated that enisamium inhibits replication of multiple subtypes of influenza A and B viruses in dNHBE cells. Further, we show that enisamium acts early in the life cycle of influenza virus, inhibiting viral RNA synthesis by an unknown mode of action, which consequently results in the inhibition of viral protein synthesis.
The study required the use of dNHBE cell to assess the antiviral activity of enisamium because the compound proved to be ineffective against influenza virus infection in MDCK cells. dNHBE cells express high concentrations of sialic acid (SA)-α2,6-galactose (Gal)-containing receptors and lesser amounts of SA-α2,3-Gal receptors,18 and functionally and morphologically recapitulate human airway epithelium. This cell system allows better understanding of compound activity at a primary site of infection (upper respiratory tract of humans) in physiologically relevant conditions. We demonstrated that enisamium at certain concentrations caused a 100-fold reduction of virus titers in dNHBE cells. Such inhibition is positively correlated with a dose-dependent drug penetration of drug into the cell. It was found that the reason why enisamium failed to exhibit antiviral activity in MDCK cells was due to its poor penetrability into those cells, whereas higher levels of the drug were found in dNHBE cells. For achieving an effective antiviral concentration of enisamium inside the cell, relatively high extracellular concentrations of this compound are needed. This pertains to cell culture assays and it is not known whether cells in the human body may require lower concentrations for compound efficacy. Since clinical efficacy has been reported,10 this suggests this may be the case. Other studies reported here demonstrated that the antiviral effect of enisamium was not linked to its cytotoxicity (the selectivity index was >20).
Although the exact mechanism of action of enisamium has yet to be identified, data presented here indicated that the drug inhibited influenza virus RNA synthesis rather than entry, uncoating, or viral release from cells. Based on the time-of-addition study, the reduction of virus yield was more pronounced when enisamium was added within 4 h after virus inoculation and the effect was slowly diminishing if the compound was added later than that time point. This is consistent with the fact that viral RNA synthesis and subsequent viral protein synthesis need to be inhibited by early drug treatment. There are other antivirals in development that directly target the influenza virus polymerase complex.19–22 However, the target of enisamium is unclear. Potentially the compound may affect a specific viral or host cell protein required for early stages of viral RNA replication. These studies are currently ongoing.
In conclusion, we have demonstrated antiviral activity of enisamium against influenza A and B viruses in dNHBE cells, supporting its reported clinical efficacy against influenza virus infections.
Acknowledgements
We are grateful to John Morrey, Donald Smee, Rajendra Mehta, Martin Schutten, and Elena A. Govorkova for valuable suggestions and helpful discussions during the conduct of these studies.
Authors’ contributions
DB, XP, MM, PD, and PT carried out the experimental work, and formulated and analyzed the data. DB and VM jointly planned experiments and wrote the manuscript.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Victor Margitich is an employee of Farmak. He provided conceptual direction, designed and supervised the overall study. He is also a coauthor of US Patents US 8,404,857 B2 and US 9,493,416 B2. DB, XP, MM, PD, PT do not have a commercial or other association that might pose a conflict of interest (e.g. pharmaceutical stock ownership, consultancy, advisory board membership, or relevant patents).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was funded by Farmak Public Joint Stock Company, Kiev, Ukraine.
References
- 1.Kamali A, Holodniy M. Influenza treatment and prophylaxis with neuraminidase inhibitors: a review. Infect Drug Resist 2013; 6: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360: 953–956. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Weekly epidemiological record, no. 44–45, 477–488, http://www.who.int/wer/2013/wer8844_45.pdf?ua=1 (2013, accessed February 2018).
- 4.Centers for Disease Control and Prevention. 2017–2018 influenza season week 2 ending January 13. Antiviral resistance, https://www.cdc.gov/flu/weekly/ (2018, accessed February 2018).
- 5.Renzette N, Caffrey DR, Zeldovich KB, et al. Evolution of the influenza A virus genome during development of oseltamivir resistance in vitro. J Virol 2014; 88: 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takashita E, Ejima M, Itoh R.et al. A community cluster of influenza A(H1N1)pdm09 virus exhibiting cross-resistance to oseltamivir and peramivir in Japan, November to December 2013. Euro Surveill 2014; 19: 1–6. [DOI] [PubMed]
- 7.Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le QM, Wertheim HF, Tran ND, et al. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362: 86–87. [DOI] [PubMed] [Google Scholar]
- 9.Zhebrovska F, Margitych V, Kostiuk G, et al. Alpha-crystalline form of carbabenzpyride Patent 8,404,857 B2, USA, 2013.
- 10.Okhapkina ЕА, Melnikova ТI, Deeva EG.et al. A prospective single-blind comparative clinical study of efficacy and safety of Amizon 0.25 g tablets manufactured by Farmak JSC in patients with influenza and influenza like illness Report of Research Institute of Influenza of North-West Branch of Russian Academy of Medical Sciences (NWB RAMS), 2010, p.1–61.
- 11.Cocking D, Cinatl J, Boltz DA, et al. Antiviral effect of a derivative of isonicotinic acid enisamium iodide (FAV00A) against influenza virus. Acta Virol 2018; 62: 191–195. [DOI] [PubMed] [Google Scholar]
- 12.Reed LJ, Muench LH. A simple method for estimating fifty percent endpoints. Am J Hyg 1938; 27: 493–497. [Google Scholar]
- 13.Lugovtsev VY, Melnyk D, Weir JP. Heterogeneity of the MDCK cell line and its applicability for influenza virus research. PLoS One 2013; 8: e75014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youil R, Su Q, Toner TJ, et al. Comparative study of influenza virus replication in Vero and MDCK cell lines. J Virol Methods 2004; 120: 23–31. [DOI] [PubMed] [Google Scholar]
- 15.Oshansky CM, Pickens JA, Bradley KC, et al. Avian influenza viruses infect primary human bronchial epithelial cells unconstrained by sialic acid alpha2,3 residues. PLoS One 2011; 6: e21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw ML, Palese P. Orthomyxoviradae: the viruses and their replication In: Knipe D, Howley P (eds) Fields’ virology. 6th ed Philadelphia, PA: Lippincott Williams & Wilkins, 2011, pp.1647–1689. [Google Scholar]
- 17.Ison MG. Antiviral treatments. Clin Chest Med 2017; 38: 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matrosovich MN, Matrosovich TY, Gray T, et al. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA 2004; 101: 4620–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 2005; 49: 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann HH, Kunz A, Simon VA, et al. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Natl Acad Sci USA 2011; 108: 5777–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih SR, Horng JT, Poon LL, et al. BPR2-D2 targeting viral ribonucleoprotein complex-associated function inhibits oseltamivir-resistant influenza viruses. J Antimicrob Chemother 2010; 65: 63–71. [DOI] [PubMed] [Google Scholar]
- 22.Jones JC, Marathe BM, Lerner C, et al. A novel endonuclease inhibitor exhibits broad-spectrum anti-influenza activity in vitro. Antimicrob Agents Chemother 2016; 60: 5504–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]