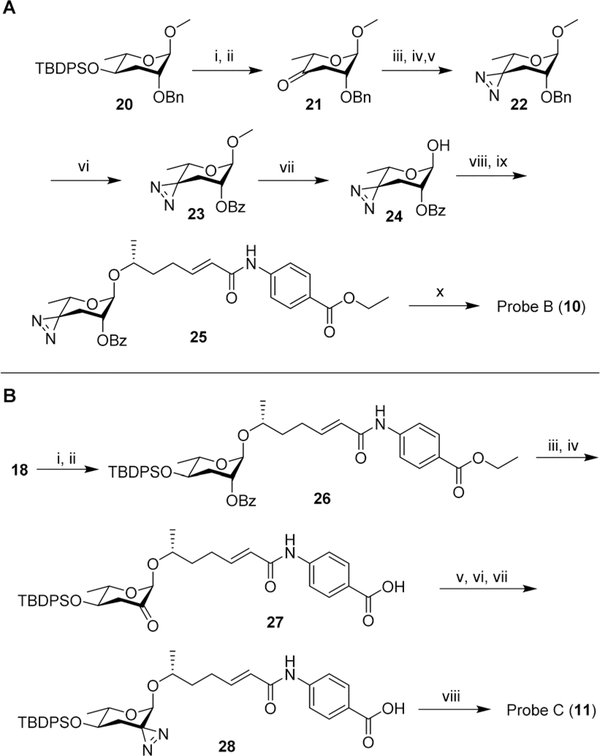
Scheme 2.
(A) Synthesis of probe B (10). (i) TBAF, THF, 25 °C, 8 h, 95%; (ii) PCC, 4 Å molecular sieves, DCM, 25 °C, 4 h, 74%; (iii) 7N NH3 in MeOH, pTsOH, MeOH, 0 °C, 3 h; (iv) NH2OSO3H, 0 °C → rt, 16 h; (v) NEt3, I2 in MeOH titration, 25 °C, 39% ~3 steps; (vi) RuCl3·H2O, NaIO4, DCM : MeCN : H2O = 1: 1: 1, 25 °C, 5 h, 72%; (vii) BBr3, DCM, −78 °C, 30 min, 71% BRSM; (viii) CCl3CN, DBU, DCM, 25 °C, 2 h; (ix) N-(6′R-hydroxy-2′E-heptenoyl)-4-aminobenzoic acid ethyl ester (prepared following previous reported method1), TMSOTf, DCM, 0 °C, 2 h, 57%~2 steps; (x) LiOH·H2O, dioxane, H2O, 60 °C, 3 h, 79%. (B) Synthesis of probe C (11).: (i) CCl3CN, DBU, DCM, 25 °C, 2 h; (ii) N-(6′R-hydroxy-2′E-heptenoyl)-4-aminobenzoic acid ethyl ester (prepared following previous reported method1), TMSOTf, DCM, 0 °C, 2 h, 15% ~2 steps; (iii) LiOH·H2O, dioxane, H2O, 60 °C, 12 h, 42%; (iv) Dess-Martin periodinane, DCM, 25 °C, 12 h, 51%; (v) 7 N NH3 in MeOH, pTsOH, MeOH, 0 °C, 3 h; (vi) NH2OSO3H, 0 °C → rt, 16 h; (vii) NEt3, I2 in MeOH titration, 25 °C; (viii) TBAF, THF, 25 °C, 8 h, 13% ~4 steps.