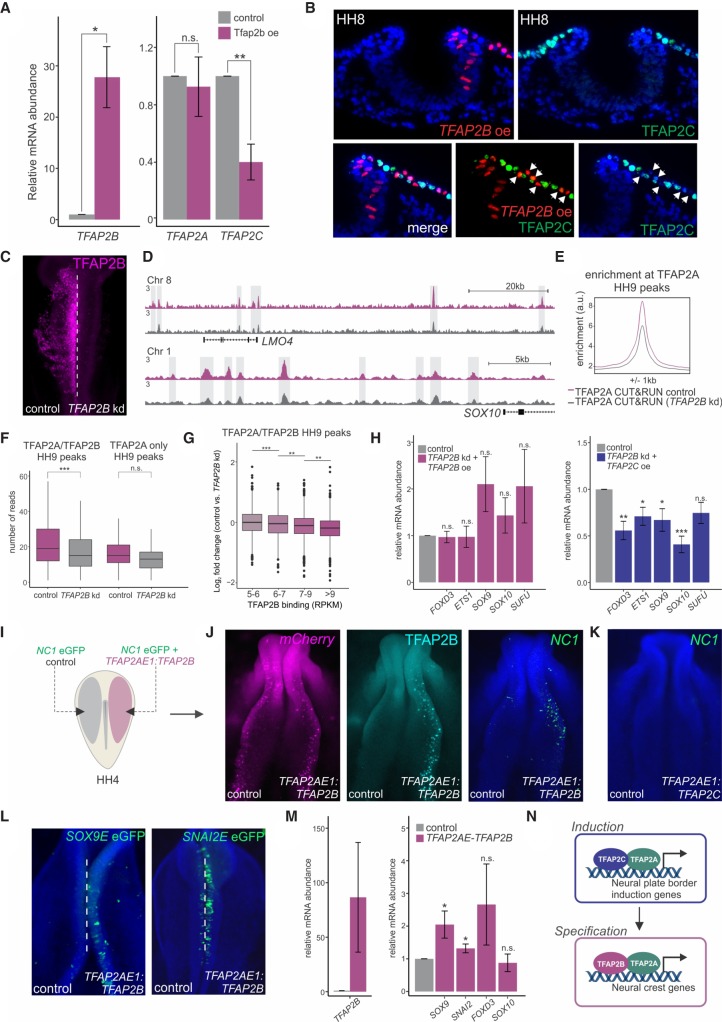
Figure 6.
Manipulation of the TFAP2 molecular switch causes premature specification. (A) Quantitative RT-PCR for TFAP2B, TFAP2A, and TFAP2C in control versus TFAP2B overexpression sides of bilaterally electroporated embryos. (B) Transverse sections displaying immunohistochemistry for TFAP2C (green) under TFAP2B overexpression (red). White arrowheads indicate a cell-autonomous decrease in TFAP2C protein levels. (C) Immunostaining for TFAP2B upon TFAP2B DsiRNA treatment. CUT&RUN for TFAP2A was performed in TFAP2B DsiRNA-treated embryos at HH9 to assess TFAP2A occupancy upon strong down-regulation of the TFAP2B protein. (D) CUT&RUN profiles for TFAP2A at the LMO4 and SOX10 loci under control versus TFAP2B knockdown conditions. (E) Profiles displaying enrichment of TFAP2A at TFAP2A peaks during specification in control versus DsiRNA-treated sides of bilaterally electroporated embryos. (F) Boxplots displaying read counts of control versus TFAP2B DsiRNA-treated TFAP2A CUT&RUN data sets at genomic regions corresponding to TFAP2B-dependent and -independent specification peaks (16,945 and 494 peaks, respectively). Statistical significance was determined via an unpaired two-tailed t-test. (G) Boxplots displaying log2 fold change in TFAP2A binding in control versus TFAP2B knockdown conditions across four levels of TFAP2B enrichment (5–6: 1185 peaks; 6–7: 1059 peaks; 7–9: 1752 peaks; more than 9: 1720 peaks). Statistical significance was determined via one-way ANOVA with Tukey's test. (H) Quantitative RT-PCR for the specification genes FOXD3, ETS1, SOX9, and SOX10 in embryos electroporated with a TFAP2B DsiRNA and rescued with a TFAP2AE1:TFAP2B or TFAP2AE1:TFAP2C expression construct. Phenotypes were surveyed at HH9. The neural marker SUFU was included as a control to ensure defects were neural crest specific. (I) Electroporation scheme for reprogramming of neural crest enhancer, NC1. HH4 embryos were bilaterally electroporated with the FOXD3 enhancer reporter NC1-eGFP, in addition to a construct driving neural crest–specific overexpression of TFAP2B (TFAP2AE1:TFAP2B). (J) mCherry reporter, Immunostaining for TFAP2B and the NC1 enhancer reporter in embryos bilaterally electroporated with TFAP2AE1:TFAP2B. Ectopic expression of TFAP2B in early presumptive neural crest cells jumpstarts the specification program, as displayed by an increase in NC1 enhancer activity. (K) NC1 enhancer reporter in embryos electroporated with TFAP2AE1:TFAP2C. Ectopic expression of TFAP2C is unable to activate NC1 enhancer activity. (L) Additional neural crest enhancers, Sox9E and Snai2E, display increased reporter activity upon premature TFAP2B expression. (M) Quantitative RT-PCR for TFAP2B, as well as the specification genes SOX9, SNAI2, FOXD3, and SOX10 in FACS-sorted mCherry+ cells from control versus TFAP2AE1:TFAP2B-electroporated sides of bilaterally electroporated embryos. Premature expression of TFAP2B results in a significant increase in expression of SOX9 and SNAI2. (N) Model for regulation of neural plate border induction and neural crest specification by TFAP2 heterodimer pairs. (A,G,L) Error bars, SE. (HH) Hamburger Hamilton; (kd) knockdown; (oe) overexpression; (kb) kilobase. (*) P ≤ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001 (for number of embryos analyzed, see also Supplemental Table S1).