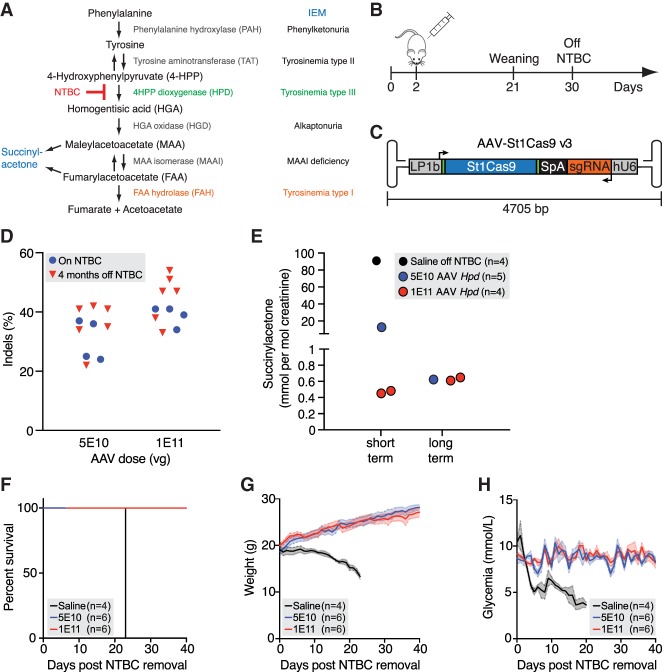
Figure 4.
In vivo genome editing using St1Cas9. (A) The tyrosine degradation pathway and associated inborn errors of metabolism (IEMs). (B) Experimental design. Neonatal (2-d-old) Fah−/− mice were injected with AAV8-St1Cas9 or saline into the retro-orbital sinus and weaned at 21 d, and NTBC was removed at 30 d of age. Mice off NTBC were sacrificed when they lost 20% of their body weight. (C) Schematic representation of the AAV-St1Cas9 v3 vector. Annotated are the liver-specific promoter (LP1b), synthetic polyadenylation sequence (SpA), and hU6 promoter. Arrows indicate the direction of transcriptional unit. (D) Neonatal Fah−/− mice were injected with either 5 × 1010 or 1 × 1011 vector genomes (vg) of AAV8-St1Cas9 v3 targeting Hpd exon 13 and sacrificed 28 d following injection or kept alive for phenotypic and metabolic studies for 4 mo post NTBC removal. Genomic DNA was extracted from whole-liver samples, and the Surveyor assay was used to determine the frequency of indels. Each dot represents a different mouse. A mouse injected with saline (−) was used as a negative control. (E) SUAC levels in urine from treated mice were determined 15 d (short term) or 4 mo (long term) following NTBC removal. Samples were collected from the indicated treatment groups over a 24-h period using metabolic cages. Number of mice per group/metabolic cage (n) and AAV doses (vg) is indicated. SUAC levels are undetectable in C57BL/6N (wild-type) mice. (F–H) Survival analysis, body weight, and glycemia following NTBC removal in treated mice. Body weight was measured daily, and glycemia was monitored in nonfasted mice. Solid lines designate the mean; and error bars are represented by shaded areas and denote SEM. See also Supplemental Figure S6.