Abstract
Inflammatory bowel disease (IBD) consists of two major idiopathic gastrointestinal diseases: ulcerative colitis and Crohn's disease. Although a significant advance has been achieved in the treatment of IBD, there remains a particular population of patients that are refractory to the conventional treatments, including the biologic agents. Studies have revealed the importance of “mucosal healing” in improving the prognosis of those difficult-to-treat patients, which indicates the proper and complete regeneration of the damaged intestinal tissue. In this regard, organoid-based regenerative medicine may have the potential to dramatically promote the achievement of mucosal healing in refractory IBD patients, and thereby improve their long-term prognosis as well. So far, studies have shown that hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) may have some beneficial effect on IBD patients through their transplantation or transfusion. Recent advance in stem cell biology has added intestinal stem cells (ISCs) as a new player in this field. It has been shown that ISCs can be grown in vitro as organoids and that those ex-vivo cultured organoids can be employed as donor cells for transplantation studies. Further studies using mice colitis models have shown that ex-vivo cultured organoids can engraft onto the colitic ulcers and reconstruct the crypt-villus structures. Such transplantation of organoids may not only facilitate the regeneration of the refractory ulcers that may persist in IBD patients but may also reduce the risk of developing colitis-associated cancers. Endoscopy-assisted transplantation of organoids may, therefore, become one of the alternative therapies for refractory IBD patients.
Keywords: Intestinal stem cells, Organoids, Ulcerative colitis, Crohn's disease, Organoid medicine
Abbreviations: ISC, intestinal stem cells; UC, ulcerative colitis; CD, Crohn's disease
1. Introduction
Inflammatory bowel disease (IBD) is a disease that is characterized by idiopathic mucosal inflammation along the gastrointestinal tract [1]. Ulcerative colitis (UC) and Crohn's disease (CD) represent the two forms of IBD. Their incidence, as well as the prevalence, is continuously increasing at the global level, including the Asian, South American, and Middle Eastern countries [2]. The treatment of IBD has dramatically improved in the past decade, mainly by the outstanding clinical effect of biologic agents such as anti-TNF-α antibodies [3]. Those therapies were targeted mostly to control the inflammation that arises at the mucosa of IBD patients. However, less attention had been paid to the recovery of the tissue damage that may manifest as intestinal ulcers. Recent clinical studies have clearly shown that “mucosal healing” is an utmost requirement to achieve long-term remission in IBD patients [4]. “mucosal healing” indicates complete restore of the mucosal structure and function. Therefore, a high demand exists for an alternative treatment that can promote tissue regeneration of refractory IBD patients.
The intestinal mucosa consists of three cell populations: lymphocytes, mesenchymal cells, and epithelial cells. In the past years, many studies have tried to use hematopoietic stem cells (HSCs) or mesenchymal stem cells (MSCs) for the treatment of IBD. Recent advance in culture methods has newly added ISCs as another candidate cell source for regenerative medicine in IBD patients. A more extensive choice of stem cell source may help to establish an effective stem cell-based alternative therapy for refractory IBD patients.
2. Old players of stem cell-based regenerative medicine in the treatment of IBD
The earliest challenge to establish stem cell-based therapy for IBD patient used HSC transplantation preceded by non-myeloablative conditioning, to rebuild or reset the host immune system [5]. In the study, HSC transplantation appeared to have the ability to induce and maintain remission of refractory CD patients. In the following periods, a series of studies further suggested some clinical benefits in HSC transplantation [[6], [7], [8], [9], [10]]. However, studies raise concerns to the high incidence of serious adverse events [11], and also to the clinical benefit itself [12,13]. From a multi-center study conducted in Europe (ASTIC trial), it was concluded that autologous HSC transplantation might not be recommended to refractory CD patients [13]. However, the possible adverse effect of cyclophosphamide used for the transplantation group has been pointed out [14]. Conversely, the most recent retrospective study concluded that autologous HSC transplantation is relatively safe and appears to be effective for treatment-resistant Crohn's disease [15]. Thus although a long time has been spent to examine the relevance of HSC transplantation to CD patients, it remains controversial whether it can be considered as a good alternative therapy for refractory CD patients.
MSCs are another stem cell population that has long been studied for its use in IBD treatment. Studies have shown that MSCs have the potential to adjust the host immune response, and at the same time promote repair of the damaged tissue. Such an effect of MSCs may come from their paracrine effect, and also from their direct engraftment to the target lesions [16,17]. Another distinct feature of MSCs is its low immunogenicity [18]. Therefore, MSCs has been used for autologous as well as allogeneic transplantation, in various disease models [19,20]. Based on such a function of MSCs, they have gone through a series of trials to treat the luminal and fistulizing type of CD. In the initial trials for luminal CD, both autologous and allogeneic transfusion of MSCs improved the disease activity of refractory CD patients, with low risk of adverse events [21,22]. Also, it has been shown that local injection of autologous MSCs to fistulizing CD can induce closure of the fistula, also with low risk of adverse events [[23], [24], [25], [26]]. A recent double-blind dose-finding study concluded that allogeneic bone-marrow-derived MSC therapy for CD associated perianal fistulas is safe and effective in terms of long-term outcome [27]. Thus, infusion or injection of MSC appears to be a safe and effective treatment for a particular population of CD patients. However, several issues remain unclear and need further studies. For example, it is not known whether the difference in the origin of MSCs may determine the clinical outcomes when they were used for the treatment of refractory CD. Also, it is not clear whether a transfusion or a local injection is better for the treatment of CD. The effect of a single MSC treatment is mostly transient and may not persist in the long term. So the frequency or the duration of the treatment may also have to be optimized.
3. Intestinal stem cells: new player in the field of stem cell-based IBD treatment
Intestinal stem cells (ISCs) reside at the bottom of the intestinal crypt and play indispensable roles in maintaining the homeostasis and the rapid renewal of the intestinal epithelium [[28], [29], [30]]. They are characterized by the expression of ISC-specific genes, such as LGR5 [31]. Also, studies have shown that loss of stem cell-specific properties may retard or disrupt the regeneration of the damaged intestinal epithelium [32,33]. Thus, it may be easy to think that transplantation of ex-vivo cultured ISCs may help promote the regeneration of the damaged intestinal epithelium in IBD patients. However, the question of how we could efficiently culture and expand donor ISCs in vitro has remained an unsolved problem for an extended period.
Series of studies by Sato et al. has provided an apparent breakthrough in this area, by their establishment of a novel culture method for ISCs [34]. They succeeded in long-term culture of ISCs by maintaining them in a 3D-structure, which was named as “organoids” [35]. The culture method required at least four growth factors, which were Wnt3a, R-Spondin-1, EGF, and Noggin. In their later studies, those factors turned out to be the indispensable components of the stem cell niche, which is supplied by the Paneth cells in vivo [36]. Therefore, the success was based on the careful in vitro reconstitution of the stem cell niche microenvironment. The culture method can be applied to grow both mice as well as human organoids [37], which can be continued infinitely for over the years. Other groups have reported that endoscopic biopsies can be used as a starting material to establish patient-derived organoids [38] and that those organoids retain the specific properties of their site-of-origin within the gastrointestinal tract [39].
Yui et al. further developed an original culture method using collagen instead of Matrigel [40]. Proving that collagen can be used as an extracellular matrix for the culture of intestinal organoids is essential for the development of organoid-based regenerative therapy, as Matrigel is not allowed for clinical use. Their recent study further showed that extracellular collagen could induce “fetalization” of organoids, which indicates a partial acquirement of the fetal intestine-specific phenotype by adult-derived intestinal organoids [41]. Such a “fetalization” is also observed in the regenerating epithelia of UC patients, thus providing the validity of using organoid cultured in collagen gels for the treatment of those patients.
Another breakthrough that has been acquired in this area was the proof that those ex vivo cultured ISCs could engraft orthotopically, and thereby contribute to the reconstruction of the damaged mucosa. A study using a DSS-colitis model showed that organoids could engraft onto the surface of the rectal ulcer when they were delivered through an intraluminal route [40]. Those donor-derived cells formed a clear crypt structure that was integrated into the recipient epithelial crypts and remained there for over months. These observations provided the evidence that ex vivo cultured ISCs can engraft and contribute to the regeneration of the damaged intestinal epithelium. Further studies showed that organoids derived from the fetal intestine or the adult small intestine are also able to engraft onto the damaged epithelium of the colon, but shows the difference in their ability to adapt to the surrounding environment through a mechanism of cell plasticity [42]. These two breakthroughs provided a sound basis to apply ex-vivo cultured ISCs for the treatment of refractory IBD. A recent study by Sugimoto et al. further confirmed that human intestinal organoids could also reconstruct the damaged mucosa of immunodeficient mice [43].
4. Expected advantages and requirements of ISC transplantation for IBD patients
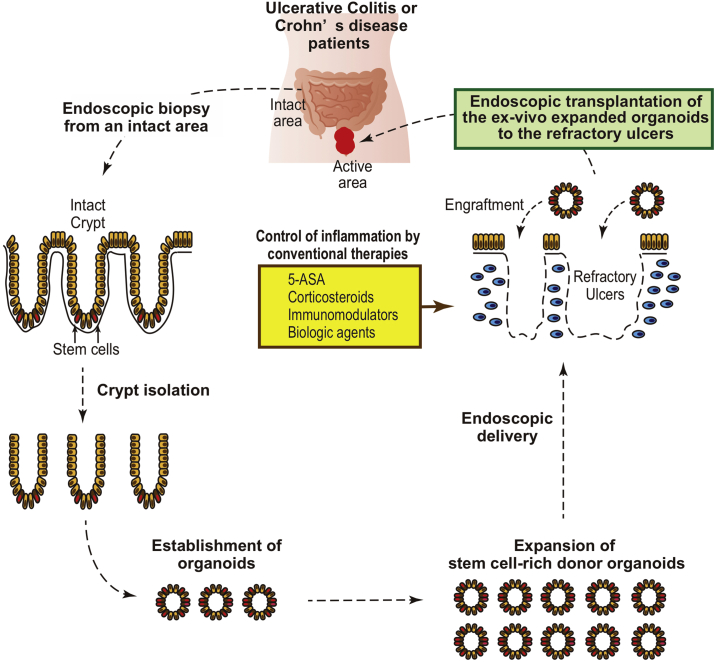
Base on those previous studies, intestinal organoids can now be considered as one of the candidate sources to repair the ulcers that may appear in refractory IBD patients. One of the strategies that may be taken for such treatment is autologous, endoscopic transplantation of ISCs (Fig. 1). ISCs can be collected from the intact lesion of a patient through the endoscopic biopsy, and then expanded in vitro by the established organoid culture method. After growing them to a desired number of cells, they can be transplanted onto the target site through an endoscopic delivery method. Presumably, it would be better to reduce or achieve good control of the mucosal inflammation before the organoid transplantation, to guarantee high engraftment efficiency.
Fig. 1.
Proposed strategy of the endoscopic intestinal stem cell transplantation for its application to IBD patients.
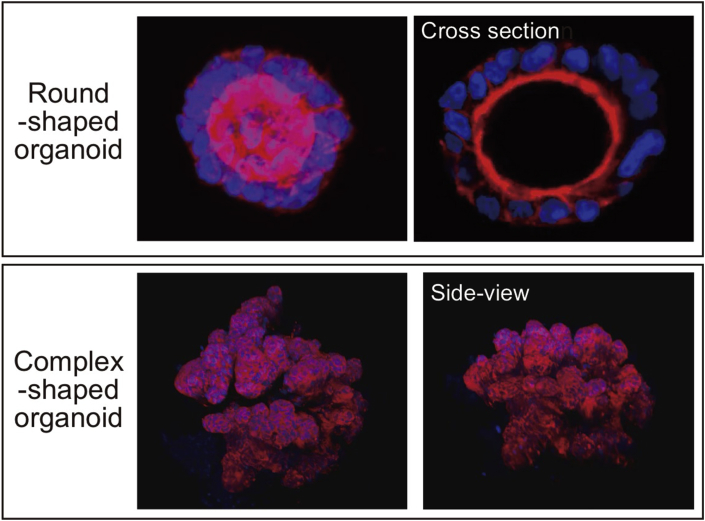
However, further researches and several technical developments are required to enable such a treatment (Table 1). At the cell culture level, we need to know whether the in vitro properties of ISCs that were derived from IBD patients is comparable to those derived from healthy donors. A recent study by our group has shown that organoids derived from active lesions of CD patients exhibit comparable growing potential compared to those from healthy donors and maintain mostly same ISC-specific gene expression profile at the single-cell level [44]. Besides, organoids can change their morphology or its ISC-content depending on the culture environment determined mainly by extracellular matrix and growth factors (Fig. 2). Further studies using transplantation model of colitis mice may reveal the optimized culture condition for transplantation therapy.
Table 1.
Remaining questions for the development of intestinal organoid transplantation therapy for IBD patients.
| Questions | Suggested solution(s) |
|---|---|
| At the cell culture level | |
| Can we expand patient derived organoids in vitro at a proliferation efficiency comparable to those derived from healthy donors? |
|
| Can we expand patient derived organoids in a completely xeno-free culture condition? Or otherwise in a fully defined culture condition? |
|
| What kind of tests should we apply for the quality assurance and quality control of donor organoids? |
|
| How could we exclude the tumorigenicity of the donor organoids? |
|
| Can we expand patient derived organoids without enhancing the risk of infectious pathogen-related adverse events? |
|
| At the cell transplantation level | |
| Is it better to deliver ISCs as an organoid, or otherwise as a cell sheet? |
|
| What kind of device is suitable to efficiently deliver organoids through an endoscopic procedure? |
|
| Is an additional technique/procedure required to promote the engraftment of the donor organoids? |
|
| Is there any host mucosal condition that is beneficial or inversely unfavorable for the engraftment of the donor organoids? |
|
| At the clinical level | |
| What will be the best index to evaluate the clinical effect of organoid transplantation? |
|
| What kind of patients is the best candidate of organoid transplantation? UC or CD? |
|
| Can we identify the donor cell derived crypts within the recipient mucosa? |
|
| Is it better to perform an allogenic organoid transplantation from a healthy donor instead of an autologous transplantation? |
|
Fig. 2.
Change in organoid morphology depending on culture condition. Human intestinal organoids show either round-shaped morphology, or otherwise a complex-shaped morphology depending on choice of extracellular matrix and on growth factor condition. Data acquired by confocal microscope system (FV3000, Olympus).
Also, to assure the safety of the treatment and avoid adverse events, validation in the quality of cultured ISCs must be established and performed. For example, the prevalence of infectious pathogens in the donor tissues should be checked, and the amplification of those pathogens during the culture period should be carefully ruled out. Also, the frequency of genomic mutations in tumor-related genes might be checked in both donor-derived tissues and in cultured organoids to exclude an increase in tumorigenic potential by the ex-vivo culture. So far, the accumulation of mutation I ISCs does not seem to increase so much by the in vitro organoid culture [45]. Also, in vivo mucosal transplantation of organoids has not yet identified the formation of organoid derived tumors [40,43].
At the cell transplantation level, a new endoscopic cell delivery system may be required. At first, we need to know what kind of extracellular matrix could be used for the transplantation process. A recent study showed that collagen, as well as fibrin glue, can be used for intestinal organoid transplantation, without showing the deleterious effect on donor organoids [46]. Delivery of the organoids through an endoscopic tool may be feasible, but another choice may be manufacturing a sheet type donor epithelial cells, and delivering them by a formerly developed device [47]. Finally, at the clinical level, we currently do not know exactly which kind of IBD patients may most benefit from such a treatment. Our opinion is to start organoid transplantation studies in refractory UC patients, whose tissue damage is persistent and carries several refractory ulcers, although their inflammation is generally under reasonable control [48,49]. For the initiation of our first-in-human study, clinical-grade culture methods and endoscopic delivery methods are both under development [50].
Then what would be the expected benefit for those patients who underwent the ISC transplantation? The first may be the improvement in mucosal healing rate that would directly lead to an improved prognosis. The second will be the possible reduction of the risk in developing colitis-associated cancer [51]. As those cancers may arise from the accumulation of genetic as well as epigenetic changes within the ISCs through their long-term exposure to the inflammatory environment, replacing such an exhausted ISCs to those fresh and lively ISCs that were grown in the most ideal and stable environment would possibly have the potential to reduce the initiation of colitis-associated cancers.
Acknowledgements
This work was supported by funding from MEXT/JSPS KAKENHI [18K15774, 18K15743, 19H03634, 19K17484]; the Research Center Network Program for Realization of Regenerative Medicine from AMED [ll18bm03041h0006, 18bk0104008h0001, 19bm0304001h0007, 19bk0104008h0002, 19bm0404055h0001]. We thank Ms. Akiko Chiyoda for helping manuscript preparation.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn W., Hanauer S. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein G.R., Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338–346. doi: 10.1002/ibd.20997. [DOI] [PubMed] [Google Scholar]
- 5.Burt R.K., Traynor A., Oyama Y., Craig R. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn disease. Blood. 2003;101:2064–2066. doi: 10.1182/blood-2002-07-2122. [DOI] [PubMed] [Google Scholar]
- 6.Burt R.K., Craig R.M., Milanetti F., Quigley K., Gozdziak P., Bucha J. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116:6123–6132. doi: 10.1182/blood-2010-06-292391. [DOI] [PubMed] [Google Scholar]
- 7.Kreisel W., Potthoff K., Bertz H., Schmitt-Graeff A., Ruf G., Rasenack J. Complete remission of Crohn's disease after high-dose cyclophosphamide and autologous stem cell transplantation. Bone Marrow Transplant. 2003;32:337–340. doi: 10.1038/sj.bmt.1704134. [DOI] [PubMed] [Google Scholar]
- 8.Scimè R., Cavallaro A., Tringali S., Santoro A., Rizzo A., Montalbano L. Complete clinical remission after high-dose immune suppression and autologous hematopoietic stem cell transplantation in severe Crohn's disease refractory to immunosuppressive and immunomodulator therapy. Inflamm Bowel Dis. 2004;10:892–894. doi: 10.1097/00054725-200411000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Oyama Y., Craig R.M., Traynor A.E., Quigley K., Statkute L., Halverson A. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552–563. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 10.Cassinotti A., Annaloro C., Ardizzone S., Onida F., Volpe D.A., Clerici M. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn's disease. Gut. 2008;57:211–217. doi: 10.1136/gut.2007.128694. [DOI] [PubMed] [Google Scholar]
- 11.Lashner B.A. Autologous hematopoietic stem cell transplantation for Crohn's disease: high risk for a high reward. Inflamm Bowel Dis. 2005;11:778–779. doi: 10.1097/01.mib.0000171286.88690.ec. [DOI] [PubMed] [Google Scholar]
- 12.Snowden J., Ansari A., Sachchithanantham S., Jackson G., Thompson N., Lobo A. Autologous stem cell transplantation in severe treatment-resistant Crohn's disease: long-term follow-up of UK patients treated on compassionate basis. Qjm Int J Med. 2014;107:871–877. doi: 10.1093/qjmed/hcu095. [DOI] [PubMed] [Google Scholar]
- 13.Hawkey C.J., Allez M., Clark M.M., Labopin M., Lindsay J.O., Ricart E. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. Jama. 2015;314:2524–2534. doi: 10.1001/jama.2015.16700. [DOI] [PubMed] [Google Scholar]
- 14.Burt R.K., Ruiz M.A., Kaiser R.L. Stem cell transplantation for refractory Crohn disease. Jama. 2016;315:2620. doi: 10.1001/jama.2016.4030. 2620. [DOI] [PubMed] [Google Scholar]
- 15.Brierley C.K., Castilla-Llorente C., Labopin M., Badoglio M., Rovira M., Ricart E. Autologous haematopoietic stem cell transplantation for Crohn's disease: a retrospective survey of long-term outcomes from the European society for blood and marrow transplantation. J Crohn’s Colitis. 2018 doi: 10.1093/ecco-jcc/jjy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaishi K., Arimura Y., Fujimiya M. Stem cell therapy for inflammatory bowel disease. J Gastroenterol. 2015;50:280–286. doi: 10.1007/s00535-015-1040-9. [DOI] [PubMed] [Google Scholar]
- 17.Williams A.R., Hare J.M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ankrum J.A., Ong J., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikane S., Ohnishi S., Yamahara K., Sada M., Harada K., Mishima K. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26:2625–2633. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- 20.Onishi R., Ohnishi S., Higashi R., Watari M., Yamahara K., Okubo N. Human amnion-derived mesenchymal stem cell transplantation ameliorates dextran sulfate sodium-induced severe colitis in rats. Cell Transplant. 2015;24:2601–2614. doi: 10.3727/096368915X687570. [DOI] [PubMed] [Google Scholar]
- 21.Duijvestein M., Vos A.W., Roelofs H., Wildenberg M.E., Wendrich B.B., Verspaget H.W. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 22.Forbes G.M., Sturm M.J., Leong R.W., Sparrow M.P., Segarajasingam D., Cummins A.G. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol H. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Ciccocioppo R., Bernardo M., Sgarella A., Maccario R., Avanzini M., Ubezio C. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 24.Cho Y., Park K., Yoon S., Song K., Kim D., Jung S. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn's fistula. Stem Cell Transl Med. 2015;4:532–537. doi: 10.5966/sctm.2014-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciccocioppo R., Gallia A., Sgarella A., Kruzliak P., Gobbi P.G., Corazza G. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin Proc. 2015;90:747–755. doi: 10.1016/j.mayocp.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Lee W., Park K., Cho Y., Yoon S., Song K., Kim D. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013;31:2575–2581. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 27.Barnhoorn M.C., Wasser M.N., Roelofs H., Maljaars J.P., Molendijk I., Bonsing B.A. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. J Crohn’s Colitis. 2019 doi: 10.1093/ecco-jcc/jjz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto R., Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci. 2005;50(Suppl 1) doi: 10.1007/s10620-005-2804-5. S34 8. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto R., Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11–21. doi: 10.1007/s00535-015-1098-4. [DOI] [PubMed] [Google Scholar]
- 30.Crosnier C., Stamataki D., Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 31.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto R., Tsuchiya K., Nemoto Y., Akiyama J., Nakamura T., Kanai T. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol-Gastr L. 2009;296 doi: 10.1152/ajpgi.90225.2008. G23 35. [DOI] [PubMed] [Google Scholar]
- 33.Gregorieff A., Liu Y., Inanlou M.R., Khomchuk Y., Wrana J.L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 34.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 35.Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 36.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2012;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T., Stange D.E., Ferrante M., Vries R.G., van Es J.H., den Brink S. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 38.VanDussen K.L., Marinshaw J.M., Shaikh N., Miyoshi H., Moon C., Tarr P.I. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middendorp S., Schneeberger K., Wiegerinck C.L., Mokry M., Akkerman R.D., van Wijngaarden S. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells. 2014;32:1083–1091. doi: 10.1002/stem.1655. [DOI] [PubMed] [Google Scholar]
- 40.Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 41.Yui S., Azzolin L., Maimets M., Pedersen M., Fordham R.P., Hansen S.L. YAP/TAZ-Dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell. 2017;22:1–37. doi: 10.1016/j.stem.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda M., Mizutani T., Mochizuki W., Matsumoto T., Nozaki K., Sakamaki Y. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752–1757. doi: 10.1101/gad.245233.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugimoto S., Ohta Y., Fujii M., Matano M., Shimokawa M., Nanki K. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. 2017;22:1–17. doi: 10.1016/j.stem.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K., Murano T., Shimizu H., Ito G., Nakata T., Fujii S. Single cell analysis of Crohn's disease patient-derived small intestinal organoids reveals disease activity-dependent modification of stem cell properties. J Gastroenterol. 2018;53:1–13. doi: 10.1007/s00535-018-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blokzijl F., de Ligt J., Jager M., Sasselli V., Roerink S., Sasaki N. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538:260. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jee J., Jeong S., Kim H., Choi S., Jeong S., Lee J. In vivo evaluation of scaffolds compatible for colonoid engraftments onto injured mouse colon epithelium. FASEB J. 2019;33 doi: 10.1096/fj.201802692RR. fj. [DOI] [PubMed] [Google Scholar]
- 47.Maeda M., Kanai N., Kobayashi S., Hosoi T., Takagi R., Ohki T. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest Endosc. 2015;82:147–152. doi: 10.1016/j.gie.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto R., Watanabe M. Investigating cell therapy for inflammatory bowel disease. Expert Opin Biol Ther. 2016;16:1–9. doi: 10.1080/14712598.2016.1177019. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto R., Watanabe M. Perspectives for regenerative medicine in the treatment of inflammatory bowel diseases. Digestion. 2015;92:73–77. doi: 10.1159/000438663. [DOI] [PubMed] [Google Scholar]
- 50.Takebe T., Wells J.M., Helmrath M.A., Zorn A.M. Organoid center strategies for accelerating clinical translation. Cell Stem Cell. 2018;22:806–809. doi: 10.1016/j.stem.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grivennikov S.I. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2012;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]