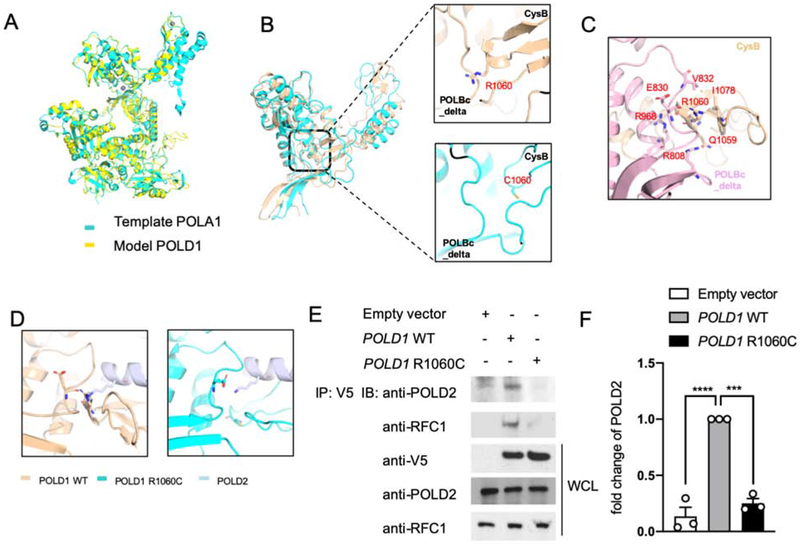
Figure 2. POLD1R1060C mutation disrupts POLD1-intrinsic and POLD1-POLD2 molecular interactions.
A. The protein structure model of full-length POLD1 using Swiss modeling based on the crystal structure of POLA1. B. Distance between the CysB motif and POLBc_delta domain in WT POLD1 and the POLD1R1060C mutant protein are measured by 10-ns snapshots. C. Detailed interface analysis of POLD1 structure. The hydrogen bonds between E830 and R1060, R968 and R1060 backbone, and R808 and Q1059 were in blue and red. D. Docking model showing the cooperation between the CysB motif and the POLBc_delta domain in recruiting POLD2 and its disruption by the R1060C mutation. E. HEK293T cells were transfected with the indicated plasmids. Twenty-four hours after transfection, cell lysates were immunoprecipitated with an anti-V5 antibody and then immunoblotted with the indicated antibodies. F. Quantitation of co-immunoprecipitated POLD2 protein by Empty vector, WT POLD1 and POLD1R1060C in E, expressed as fold change compared to that of WT POLD1 (n=3; open circles). Results represent means ± S.E.M. ***, p<0.001; ****, p<0.0001, by one-way ANOVA with Bonferroni post-test analysis.