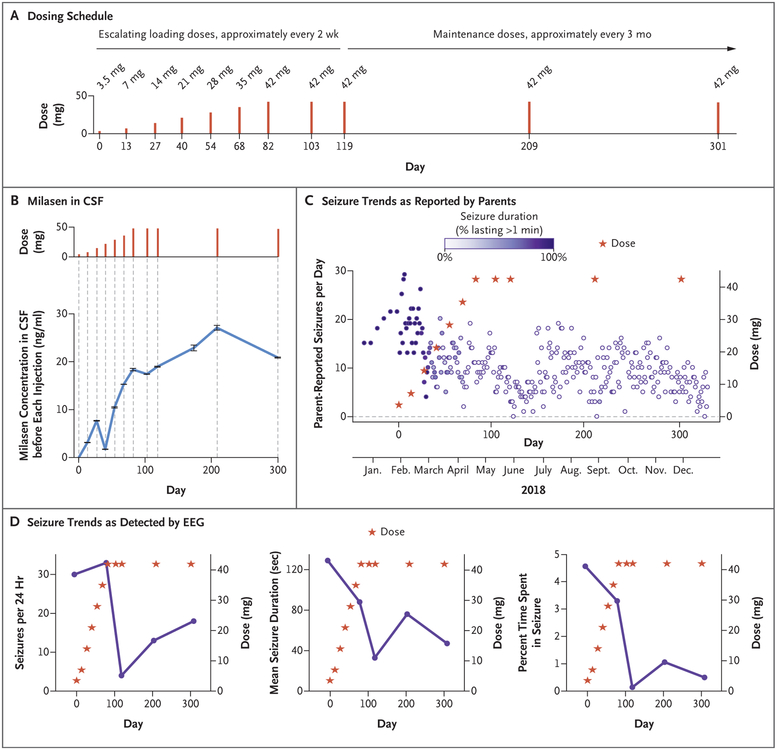
Figure 3. N-of-1 Clinical Study.
Panel A shows the dosing schedule. (Additional details are provided in Fig. S14A.) Panel B shows the concentration of milasen in cerebrospinal fluid (CSF) before each administration (trough). An additional measurement of the concentration in CSF was obtained at day 174 (without concurrent dose administration). I bars indicate the minimum and maximum values of duplicate measurements. Trough levels rose steadily in a dose-proportional fashion until day 40, at which point they dropped to 1.7 ng per milliliter and then resumed their rise with repeated dosing up to a plateau of 18 to 27 ng per milliliter. The dip at day 40 may have been due to a CSF leak, given its coincident timing with a post–lumbar puncture headache after the previous dose. A similar plateauing of CSF trough levels was observed in a previous study of intrathecally delivered nusinersen (9 to 11 ng per milliliter after four repeated doses of 12 mg).8 Panel C shows the trends in seizure frequency and duration as reported in a seizure diary recorded by the parents. Seizures were all of the same type: sudden startle followed by uncontrollable, untriggered laughter that was different from the patient’s natural laugh, at times accompanied by an increase in the nonspecific repetitive hand movements she had at baseline. Panel D shows the trends in seizure activity as detected by electroencephalography (EEG). In a comparison of the means of the initial two recordings and the subsequent three recordings, the daily seizure count, seizure duration, and percent cumulative time spent in seizure decreased by 63% (from 31.5 to 11.7 per day), 52% (from 108 seconds to 52 seconds), and 85% (from 3.9% to 0.6%), respectively.