Figure 9.
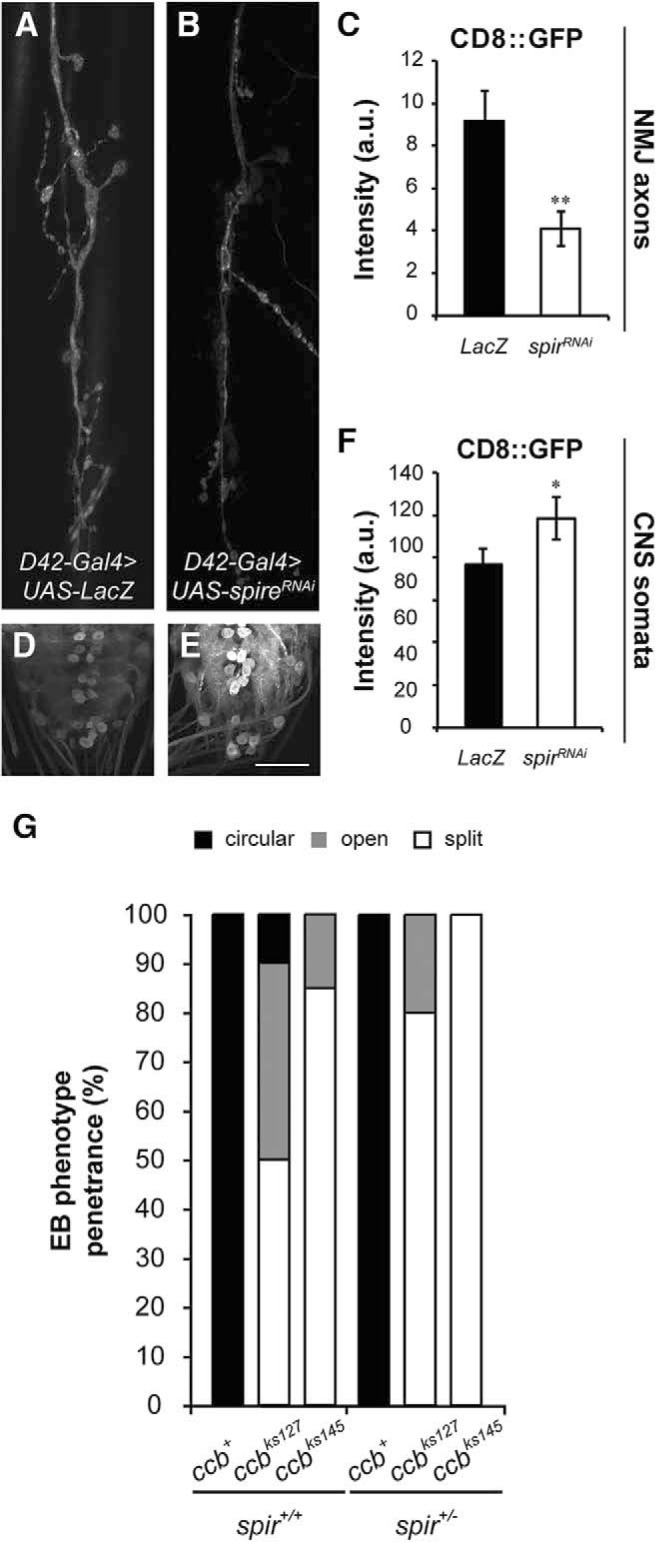
spire loss of function impairs axonal transport and enhances the ccb phenotype in the EB. A, B, NMJ of larval motor neurons innervating muscle fibers 6 and 7. Vesicular CD8::GFP accumulated at the larval NMJ is detected by the GFP signal for control (A; UAS-CD8::GFP/UAS-LacZ, D42-Gal4/+) and spireRNAi expressing (B; UAS-CD8::GFP/+, D42-Gal4/UAS-spireRNAi) animals. C, Quantification of the GFP signal in (A, B) reveals a significant reduction of vesicle-based axonal transport of CD8::GFP to the larval NMJ in motor neurons expressing the spireRNAi (C; Kruskal–Wallis, p = 0.0094). D, E, Accumulation of CD8::GFP in somata of the larval ventral ganglia. CD8::GFP accumulated in somata of the CNS motor neurons is detected by the GFP signal for control (D; UAS-CD8::GFP/UAS-LacZ, D42-Gal4/+) and spireRNAi expressing (E; UAS-CD8::GFP/+, D42-Gal4/UAS-spireRNAi) animals. F, Quantification of the GFP signal (D, E) shows significant retention of CD8::GFP in the soma of motor neurons expressing the spireRNAi (F; Kruskal–Wallis, p = 0.0263). G, Quantification of the ccb phenotype in the ellipsoid body of wild-type and heterozygous mutant background for spire. Flies carrying the ccb mutations (ccbks127 or ccbks145) are grouped into three different categories based on the EB morphology, “circular” for flies with a morphologically normal EB, “open” for flies with an EB where the ventral region is not completed, and “split” for flies with two independent EBs, each of them located on opposite sides of the midline and formed only by axons from ipsilateral R-neurons. The proportion of each phenotype is calculated as the percentage of the total number of flies analyzed for each genotype. The data show a significant increase in the severity of these aberrations on a spire mutant background (spir1/+) compared with controls (spir+/+). Scale bars: A, B, 12 μm; D, E, 50 μm. Error bars indicate SEM. *p < 0.05, **p < 0.01; n = 3 (C, F) and 25, 35, 40, 20, 20, 20 (G) per group.
