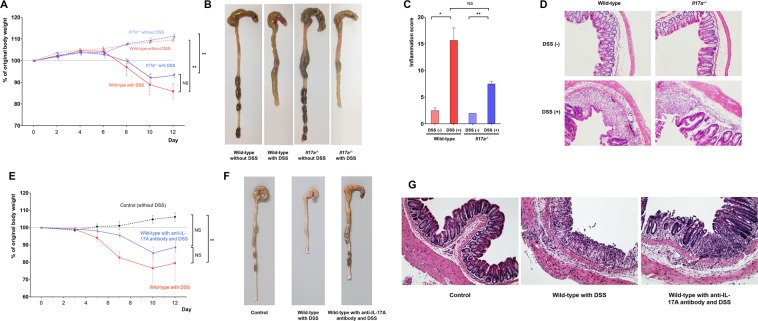
Figure 2.
DSS-induced colitis model. Bodyweight changes (A), representative gross photos of the colorectal area (B), inflammation scores (C), and representative microscopic images of histopathological examination (x200) (D) after administration of DSS for wild-type and Il17a−/− mice (wild-type mice without DSS: n = 5; wild-type mice with DSS: n = 8; Il17a−/− mice without DSS: n = 7; Il17a−/− mice with DSS: n = 9). Bodyweight changes (E), representative gross photos of the colorectal area (F), and representative microscopic images of histopathological examination (x200) (G) according to administration of anti-IL-17A antibody (wild-type mice without DSS: n = 3; wild-type mice with DSS: n = 3; wild-type mice with anti-IL-17A antibody and DSS: n = 4). In the anti-IL-17A antibody group, 100 μL of secukinumab was injected intraperitoneally three times per week starting two weeks before DSS administration, continuing until the end of the experiment. Bars represent standard errors. * P < 0.05, ** P < 0.01. DSS, dextran sulfate sodium; NS, not significant.