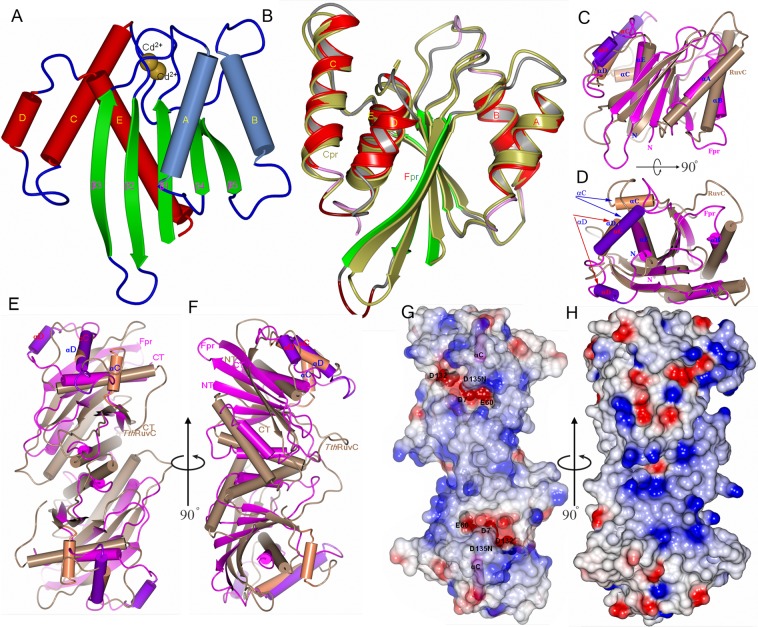
Figure 1.
Overall structure of the fowl poxvirus resolvase (Fpr). (A) Structure of Fpr with the secondary structure elements labeled. (B) Superposition of Fpr and Cpr monomers based on the central five β-strands. (C) Superposition of Fpr and RuvC from T. thermophiles (TthRuvC) monomers, highlighting that two proteins have different αC and αD helices (colored differently from the rest of the monomers and indicated by arrows). αB and the preceding loop in TthRuvC are longer than the counterparts in Fpr, while αC in Fpr superposes onto αD of TthRuvC but is much longer. (D) 90° rotation of C around a horizontal axis. (E,F) Superposition of Fpr and RuvC dimers showing the different shape of Fpr and TthRuvC, F is 90° rotation of E around a vertical axis. (F) Electrostatic surface representation of the Fpr dimer. The negative surface (in red) formed by the active site residues can be seen at the bottom of the DNA binding grooves. The αC helices are shown in magenta. (H) 90° rotation of G around a vertical axis, showing strong positive potentials (blue) on the side of active site of the Fpr dimer. The orientations of G and H are same as E and F respectively.