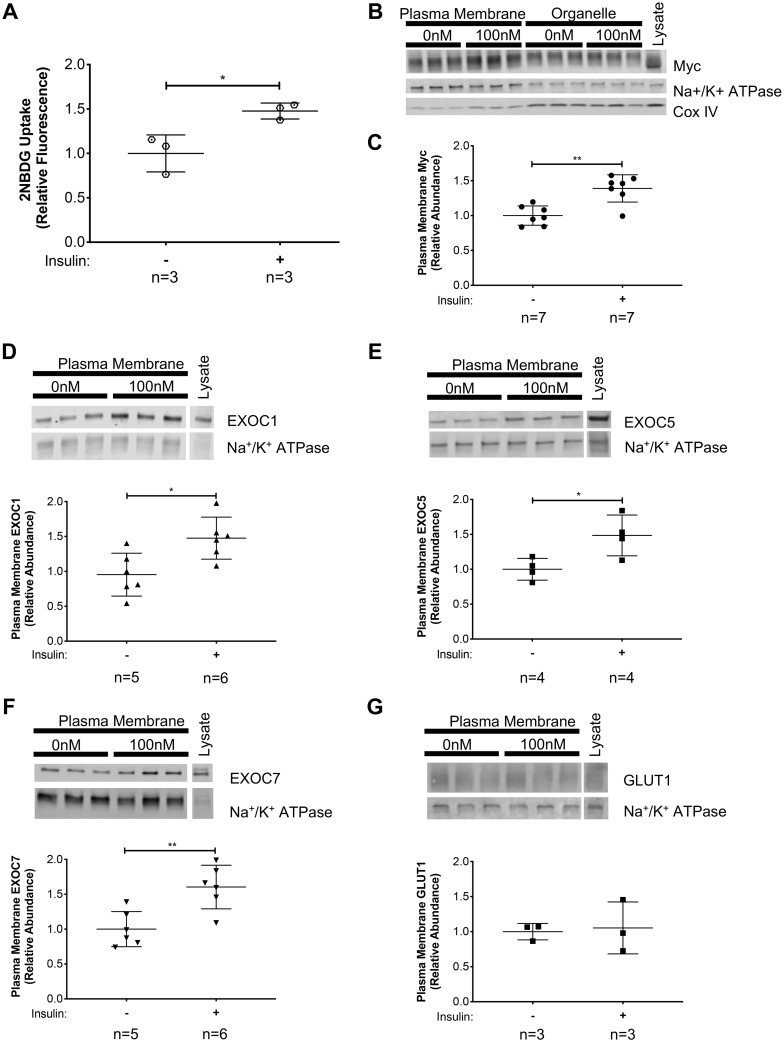
Fig. 1.
Exocyst components and glucose transporter type 4 (GLUT4) are translocated to the plasma membrane of skeletal myoblasts following insulin stimulation. A: insulin-stimulated glucose uptake in L6-GLUT4myc myoblasts was verified using the fluorescent glucose analog, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG). B–G: L6-GLUT4myc myoblasts were stimulated with 100 nM insulin for 10 min. Plasma membrane proteins were collected via subcellular fractionation and analyzed by Western blotting. B: representative images of Western analyses of subcellular fractionations. Analysis is shown of protein enrichment in subcellular fractions, where GLUT4myc distribution in subcellular fractions was detected, along with the plasma membrane protein Na+-K+-ATPase and the mitochondrial protein CoxIV. C: quantification of plasma membrane GLUT4myc, as detected by a myc-tag-specific antibody, normalized to measured levels of the membrane protein Na+-K+-ATPase. D–G: quantification of EXOC1, EXOC5, and EXOC7, and GLUT1 protein levels, respectively, in the plasma membrane fractions of insulin-stimulated myoblast cells. Signal intensities for myc, EXOC1, EXOC5, EXOC7, and GLUT1 were normalized to plasma membrane-specific Na+-K+-ATPase levels. Images and graphs presented are representative of data from 3 independent experiments. Values are means ± SD in relative units (vs. untreated cells); n, no. of replicates. *P < 0.05 and **P < 0.01, compared with untreated control.