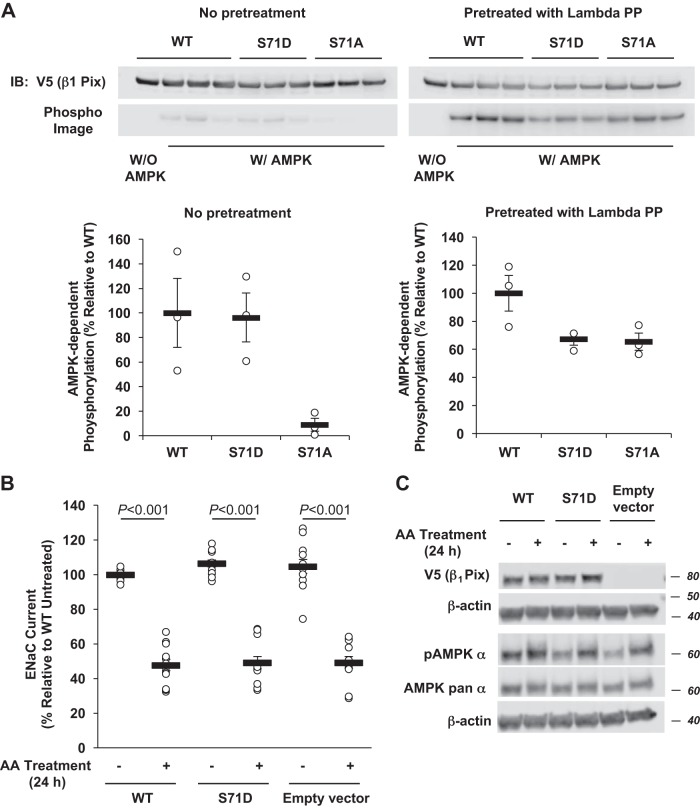
Fig. 4.
Effects of a phosphomimetic p21-activated kinase-interacting exchange factor-β1 β1Pix-S71D mutation on phosphorylation and epithelial Na+ channel (ENaC) regulation by AMP-activated protein kinase (AMPK). A: V5-tagged β1Pix [wild type (WT), S71D, or S71A] constructs were transiently transfected into human embryonic kidney-293 cells, immunoprecipitated using an anti-V5 agarose affinity gel, and treated with (right) or without (left) λ-protein phosphatase (lambda PP) to remove any preexisting phosphorylation. Purified V5-tagged β1Pix proteins were then exposed to recombinant AMPK holoenzyme or buffer alone and [γ-32P]ATP. After SDS-PAGE and transfer to a nitrocellulose membrane, immunoblot (IB) analysis and exposure to the phosphorimager were performed on the same membrane for quantitation. B: V5-tagged β1Pix-WT, β1Pix-S71D, or empty vector was transiently expressed in mpkCCDc14 cells. Two days after transfection, measurements of equivalent short-circuit current were conducted in the absence (−) or presence (+) of combined (AA) treatment with the AMPK activators 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (1 mM) and A769662 (100 μM) for 24 h. C: representative IB result of V5-tagged β1Pix, phospho-AMPK, and AMPK pan α-subunit in total lysates from mpkCCDc14 cells expressing V5-tagged β1Pix-WT, β1Pix-S71D, or empty vector after 24-h treatment with or without AMPK activators. Data are means ± SE; n = 4 separate experiments, with a total of 12 measurements/condition. P values are shown for relevant comparisons if significant.