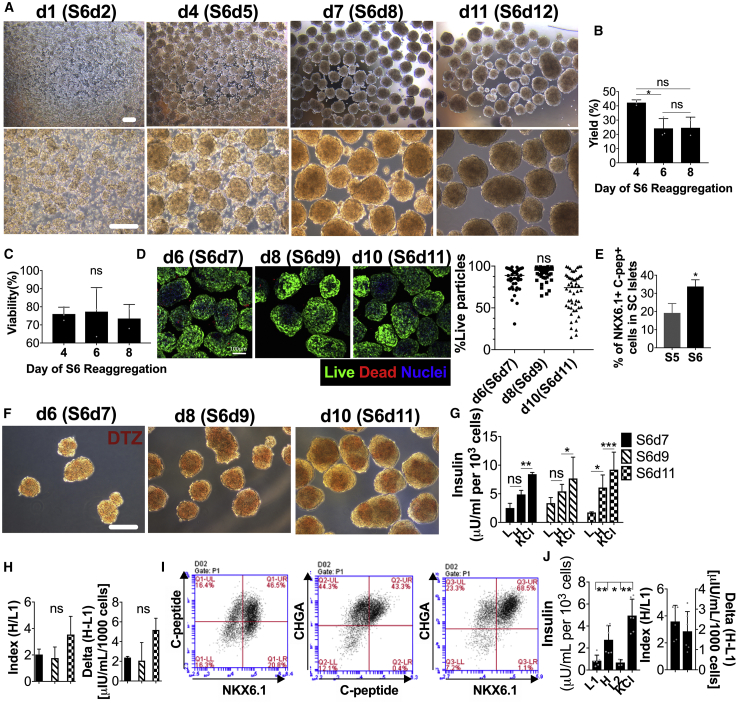
Figure 1.
In Vitro Assessment of Stage-6 SC Islets Reaggregated from Cryopreserved End-of-Stage-5 Cells
(A–C) Phase-contrast images (A) of stage-6 day-1 SC cells thawed and reaggregated in spinner flasks for 1 (S6d2) to 11 (S6d12) days at different magnifications. Scale bars, 200 μm. Live cell yield (B) and viability (C) of SC islets post thawing during reaggregation in spinner flasks (n = 3 reaggregation batches) assessed using trypan blue exclusion and automated cell counting.
(D) Confocal images (maximal projection of 150 μm-thick z stacks) and quantification of live/dead stained stage-6 day-7 (S6d7), S6d9, and S6d11 after thawing and reaggregation. Scale bar, 100 μm.
(E) β cell purity in SC islets as percentage of NKX6.1+C-peptide+ cells at the end of S6 reaggregation compared with the end of S5 before cryopreservation (n = 3 differentiation batches).
(F–H) Dithizone (DTZ) staining (F) and static GSIS functionality (G and H) of S6d7, S6d9, and S6d11 SC islets as GSIS absolute insulin secretion (G), index and delta (H) (n = 3 wells per condition assayed). SC islets were stimulated sequentially with 2.8 mM glucose (L), 20 mM glucose (H), and 30 mM KCl solutions. Scale bar, 200 μm.
(I and J) Characterization of six independent differentiation batches of S6 SC islets reaggregated from cryopreserved S5 cells assessed by flow cytometry (I) and by GSIS (J) (sequential stimulation with 2.8 mM glucose [L], 20 mM glucose [H], 2.8 mM glucose [L], and 30 mM KCl solutions). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.01. ns, no significant differences found.
All error bars are derived from standard deviations.