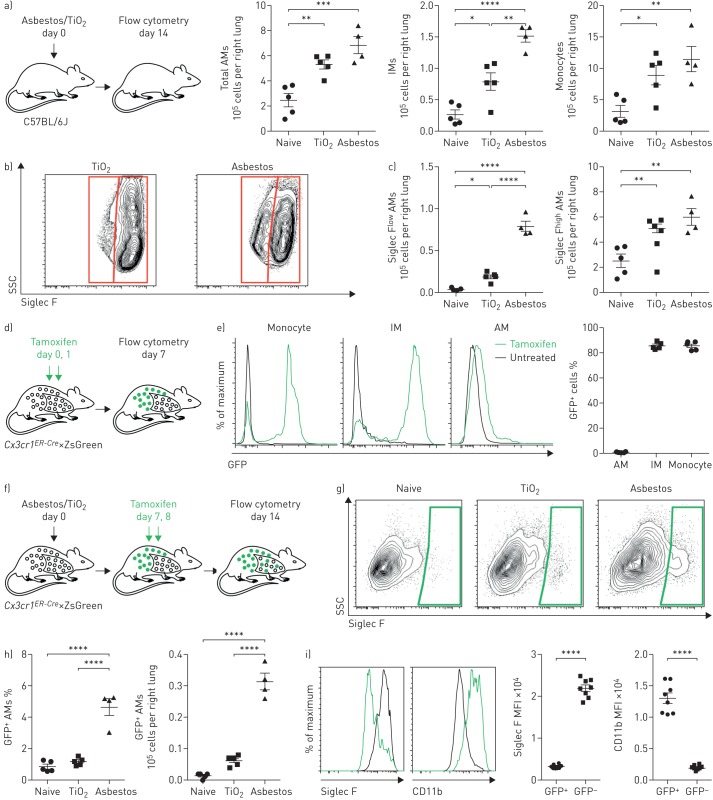
FIGURE 1.
Exposure to asbestos or TiO2 is distinguished by the recruitment of monocyte-derived alveolar macrophages (Mo-AMs) to the lung. IM: interstitial macrophage; SSC: side scatter; FSC: forward scatter; GFP: green fluorescent protein; MFI: median fluorescence intensity. a) Mice were administered crocidolite asbestos or TiO2 (both at 100 µg intratracheally), and monocyte and macrophage populations were quantified by flow cytometry 14 days later (see supplementary figure S1a and b for gating strategy and quantification of other myeloid cell populations). b) Representative contour plots gated on AMs (CD64+Siglec F+) from asbestos- or TiO2-treated animals. c) Quantification of Siglec Flow and Siglec Fhigh AMs from naive, TiO2- or asbestos-exposed animals according to gating in (b). d) Schematic of the experimental design for (e). e) Cx3cr1ER-Cre×ZsGreen mice were treated with tamoxifen, and the percentage of GFP+ classical monocytes, IMs and AMs was assessed by flow cytometry. Representative histograms showing GFP expression are shown. f) Schematic of the experimental design for (g) and (h): lineage tracing system to track the ontogeny of AMs after intratracheal administration of asbestos or TiO2. Cx3cr1ER-Cre×ZsGreen mice were treated with asbestos or TiO2 and tamoxifen was administered as two boluses at days 7 and 8. The number of GFP+ AMs was analysed 7 days later. g) Representative contour plots and h) quantification of GFP+ Mo-AMs after asbestos or TiO2 exposure. i) Representative histograms and MFI demonstrating expression of Siglec F and CD11b on Mo-AMs 14 days after exposure to asbestos. All data are presented as mean±sem. n=4–5 mice per group. One-way ANOVA with the Tukey–Kramer test for multiple comparisons. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. Representative data from two independent experiments.