Abstract
Aims: Molecular profiling is important for cancer diagnosis and treatment. For many advanced stage lung cancer patients, cytology specimens may be the only materials available for molecular testing. The aim of this study is to evaluate the utility of Next-Generation Sequencing (NGS) of cytology specimens for the molecular profiling of lung adenocarcinoma. Methods: NGS was performed on cell blocks of pleural effusions and fine-needle aspiration (FNA) samples of lung adenocarcinoma to determine the mutation status of EGFR, KRAS, PIK3CA, BRAF, ALK, PDGFRA, and DDR2. Then, quantitative Real-Time PCR (qPCR) was performed and the results were compared to those of NGS. Next, NGS was performed on available histological specimens from the same patients. Last, DNA Quality Index analysis was performed to further explore the applicability of using cytology samples as the source for NGS. Results: NGS detected mutations in EGFR, PIK3CA, and KRAS. NGS and qPCR results showed high concordance. NGS exhibited advantages over qPCR in detecting non-hotspot mutations and providing accurate information for allele sequence and mutation frequency. NGS of cytological and histological samples from the same patients showed high concordance. DNA Quality Index analysis showed that DNA extracted from cell blocks of pleural fluid was of similar quality compared to FFPE tissue blocks. Conclusions: NGS can be successfully performed on both FNA and pleural fluid samples from lung adenocarcinomas. The high quality DNA of FFPE cell block of pleural effusion makes it the first choice for molecular profiling, especially when cytology specimens are the only available samples for molecular profiling.
Keywords: Cytology, lung adenocarcinoma, next-generation sequencing (NGS), fine-needle aspiration, pleural effusion
Introduction
Lung cancer is a leading cause of cancer related death worldwide [1-3]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers, and NSCLC patients are often diagnosed at advanced stage with poor prognosis [4-6]. Adenocarcinoma is the most common subtype of NSCLC, constituting approximately 40% of all NSCLC cases [5]. In the era of precision medicine, molecular testing plays a critical role in diagnosis and clinical decision-making [7,8]. When mutations of EGFR or rearrangements of ALK and ROS1 are detected in patients with lung adenocarcinoma, individualized therapy can be applied [4,9,10]. In NSCLC patients with EGFR mutations treated with the EGFR tyrosine kinase inhibitors gefitinib or erlotinib, response rates and progression-free survival showed statistically significant improvement [10-12]. NSCLC patients with ALK rearrangements can benefit from the targeted agent, crizotinib, which has shown to be superior to standard first-line pemetrexed-plus-platinum chemotherapy in patients with previously untreated advanced ALK-positive NSCLC [13].
Cytology specimens are increasingly recognized as potential sources for molecular testing [14,15]. In some cases, cytology specimens may be the only materials available. However, the regular use of cytology samples in molecular testing remains challenging due to a paucity of tumor cells and/or a high degree of cellular heterogeneity [16]. Therefore, it is important to select a molecular test method that takes these factors of cytology specimens into account.
Next-Generation Sequencing (NGS) technology is a cost-effective method that identifies clinically actionable mutations across various genes in a high-throughput manner. It can be performed on three levels: disease-targeted gene panels, exome sequencing, and genome sequencing [17]. It can detect not only somatic mutations in hematopoietic and solid tumors, but also constitutional mutations in genes associated with inherited cancer predisposition syndromes [4]. A recent study demonstrated a 100% concordance rate between the results of NGS and conventional platforms, such as tetra-primer amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) and fluorescence in situ hybridization (FISH) in lung adenocarcinomas, using surgical pathological specimens [1]. It was reported NGS could be performed successfully on DNA extracted from smears prepared from FNA [9,15,18]. Qiu et al. reported that NGS also could be performed using paraffin-embedded cytology samples obtained from lymph node FNA [4].
In this study, we aimed to evaluate NGS using the Ion Torrent technology for detecting mutations in multiple genes related to lung adenocarcinomas (EGFR, KRAS, PIK3CA, BRAF, ALK, PDGFRA, and DDR2) in FFPE cell blocks of fine-needle aspiration (FNA) and pleural fluid specimens.
Methods
Patients and samples
Eighteen patients diagnosed with lung adenocarcinoma at West China Hospital of Sichuan University (Chengdu, China) between March 2014 and June 2015 were enrolled in this study. Informed consent was obtained from all participants or from their guardians. The cytology specimens were obtained either by FNA or from pleural fluids. Cytological smears and paraffin embedded cell block were prepared for each case. All experimental protocols were approved by West China Hospital of Sichuan University and all methods in this study were carried out in accordance with relevant guidelines and regulations. The cytological diagnoses were confirmed by immunocytochemistry and/or histology. Briefly, the samples were stained with anti-TTF-1 (clone 8G7G/1, ultraView Universal DAB Detection Kit, Ventana Medical Systems, Tucson, AZ, USA) and anti-Napsin A (polyclonal antibody, Bond Polymer Refine Detection, Leica Biosystems Ltd, Newcastle, United Kingdom) following the manufacturers’ instructions.
An experienced pathologist assessed the neoplastic cellularity of each sample using a method described previously by Wei et al. [15]. Briefly, 10-20 of the highest cellularity areas in hematoxylin and eosin (H&E) stained cell block slides were selected and the percentage of tumor cells was calculated. To ensure consistency of tumor cell estimation, all nucleated cells including epithelial cells, stromal cells, and inflammatory cells (neutrophils, eosinophils, and basophils) were counted as the denominator.
Genomic DNA extraction and quantitation
Genomic DNA was extracted from five consecutive 5-μm slides of formalin-fixed, paraffin-embedded (FFPE) cell blocks using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA concentration was measured using a genomic DNA quantitative detection kit (Beijing ACCB Biotech Ltd., Beijing, China) on a CFX96 RT-PCR instrument (Bio-Rad, Hercules, CA, USA). DNA samples were stored at -20°C before detection of lung cancer-associated genes.
Mutation analysis by next-generation sequencing
Libraries were prepared using the NextDaySeq Lung panel (Beijing ACCB Biotech Ltd., Beijing, China) on the Ion TorrentTM System according to the manufacturer’s instructions. Briefly, 16 exons of seven genes-EGFR exon 18/19/20/21; KRAS exon 2/3; PIK3CA exon 9/20; BRAF exon 11/15; ALK exon 23/25; DDR2 exon 18; and PDGFRA exon 12/14/18-were amplified using pooled primer pairs followed by ligation with adaptors and barcodes. After purification, libraries were quantified using a Qubit dsDNA HS Assay Kit on a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), diluted to a concentration of 3 ng/μl and pooled. The library pool was sequenced using the Ion Torrent PGMTM system according to the manufacturer’s instructions. Briefly, the library pool was clonally amplified in an emulsion PCR reaction using ion sphere particles on the Ion OneTouch 2 instrument, and template-positive ion sphere particles were enriched on the Ion OneTouch ES (Thermo Fisher Scientific, Waltham, MA, USA). Sequencing was performed using an Ion PGMTM Sequencing Supplies 200 v2 Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. The libraries were loaded onto Ion 318 chips to generate sequencing data with a minimum depth of 10,000 ×. Sequence variants were identified and annotated using a proprietary DanPA bioinformatics pipeline (Beijing ACCB Biotech Ltd., Beijing, China).
Mutation analysis by quantitative real-time PCR
Mutation profiles of EGFR, KRAS, PIK3CA, and BRAF were detected using a Human EGFR Gene Mutation Detection Kit, a Human KRAS Gene Mutation Detection Kit, a Human BRAF Gene Mutation Detection Kit, and a Human PIK3CA Gene Mutation Detection Kit, respectively (all kits were obtained from Beijing ACCB Biotech Ltd., Beijing, China). The tests cover 63 hotspot mutations including 45 in exons 18, 19, 20, and 21 of EGFR; 12 in exons 2 and 3 of KRAS; 5 in exons 9 and 20 of PIK3CA; and BRAF V600E. Quantitative real-time PCR (qPCR) was performed on a Mx3000P qPCR instrument (Agilent, Santa Clara, CA, USA) with the following settings: 95°C for 10 min, 40 cycles of 95°C for 15 seconds, and 60°C for 1 min. Results were interpreted according to the manufacturer’s instructions.
Sanger sequencing
Sequencing was conducted bidirectionally using the same primers as for the initial amplification reaction, using an ABI Prism BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). The sequencing primer extension reactions were analyzed on an ABI 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
DNA quality index analysis of clinical specimens
The DNA quality of 211 consecutive surplus specimens-including FFPE tissue blocks, FFPE cell blocks of pleural fluid, FFPE cell blocks of FNA samples, and freshly centrifuged pleural effusion samples-was evaluated. The DNA Quality Index and amplifiable DNA concentrations were determined using a DNA Quality Index Kit (Beijing ACCB Biotech Ltd., Beijing, China) according to the manufacturer’s instructions. Briefly, DNA was assessed with a two-channel (FAM and HEX) assay on a CFX96 RT-PCR instrument (Bio-Rad, Hercules, CA, USA). The two channels amplify a long DNA fragment and a short DNA fragment, respectively. Two calibration curves were generated using control DNA. The DNA Quality Index was calculated as the FAM/HEX signal ratio.
Results
Molecular profiling using NGS
The 18 cytology specimens included eight pleural fluids (Figure 1) and ten FNA specimens (five lymph nodes, and one each of lung mass, bone lesion, thyroid nodule, subcutaneous mass, and cardia mass). The cancer cellularity of these samples ranged from 1% to >90%. The three samples with the lowest cellularity-samples #1 (1%), #17 (2%), and #8 (20%)-were all pleural fluid samples. All other samples had greater than or equal to 30% cancer cellularity. FNA samples generally had higher cellularity (median: 80%) compared to pleural fluid samples (median: 30%), consistent with the fact that pleural fluids contain more benign cells.
Figure 1.
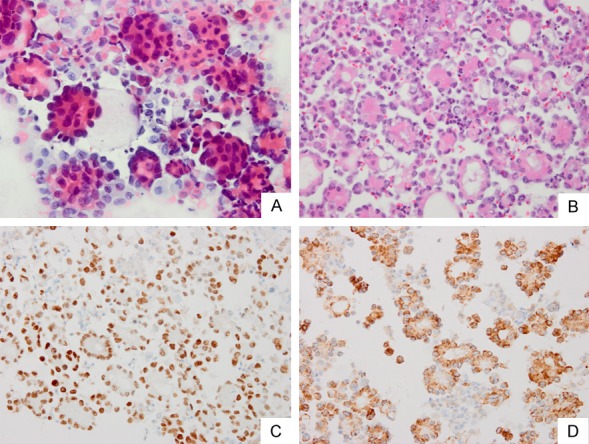
Body fluid of (A) smear and (B) cell block slide from a patient with lung adenocarcinoma. (C) In order to confirm the adenocarcinoma from lung, staining was done with (C) TTF-1 (D) and Napsin A to mark the carcinoma cells.
The DNA concentrations ranged from <0.1 ng/μL to 115.98 ng/μL (Table 1).
Table 1.
Patient clinicopathological characteristics
| NO | Tissue source | Age | Gender | Cancer Cellularity, % | TTF-1, NapsinA | Tissue specimens | DNA, ng/μL |
|---|---|---|---|---|---|---|---|
| 1 | Pleural fluid | 44 | F | 1 | +, - | Y | 44.16 |
| 2 | FNA (Lymph node) | 60 | M | 70 | +, + | N | 2.13 |
| 3 | FNA (Bone lesion) | 50 | M | 30 | +, + | N | 2.34 |
| 4 | FNA (Lymph node) | 69 | F | >90 | +, + | N | 2.48 |
| 5 | FNA (Lymph node) | 46 | F | 60 | -, + | N | 0.07 |
| 6 | Pleural fluid | 37 | M | 30 | +, + | N | 8.69 |
| 7 | FNA (Lymph node) | 37 | M | 90 | +, + | N | 4.37 |
| 8 | Pleural fluid | 48 | M | 20 | +, N | N | 25.92 |
| 9 | Pleural fluid | 27 | F | 30 | +, + | N | 50.66 |
| 10 | FNA (Lung mass) | 56 | M | 80 | +, - | Y | 26.89 |
| 11 | Pleural fluid | 29 | F | 40 | +, + | N | 3.93 |
| 12 | Pleural fluid | 27 | M | >90 | +, + | N | 101.4 |
| 13 | FNA (Lymph node) | 62 | F | 80 | +, + | N | 1.16 |
| 14 | FNA (Thyroid nodule) | 58 | F | 80 | +, + | N | 0.01 |
| 15 | FNA (Subcutaneous mass) | 45 | F | 60 | N | Y | 1.04 |
| 16 | Pleural fluid | 55 | F | 40 | N | Y | 64.51 |
| 17 | Pleural fluid | 62 | M | 2 | +, + | N | 115.98 |
| 18 | FNA (Cardia mass) | 64 | M | 80 | +, + | N | 36.86 |
Abbreviations: FNA, fine-needle aspiration; F, female; M, male; TTF-1, thyroid transcription factor-1; Napsin A, novel aspartic proteinase A; Y, Cytological diagnosis is confirmed by biopsy or surgical pathology; N, Not done or there is no matching histological sample; +, The marker is stained positive; -, The marker is negative.
DNA concentration was generally higher in pleural specimens than in FNA samples (median concentration of 47.41 ng/μL vs. 2.24 ng/μL, respectively). Of 18 cases, two FNA samples were not subjected to any further analysis due to low DNA concentration (<0.1 ng/μL, samples #5 and #14).
We used a NextDaySeq Lung panel (Beijing ACCB Biotech Ltd., Beijing, China), which is based on Ion Torrent Technology, to examine the mutation status of EGFR, KRAS, PIK3CA, BRAF, ALK, PDGFRA, and DDR2. This panel covers 16 full exons of these seven genes. Of the 16 samples that were subjected to NGS analysis, one sample (sample #11) failed quality control due to inadequate reads. Therefore, 15 samples were successfully sequenced, with a median coverage >1000 × and >80% of amplicons with 500 × coverage (data not shown).
Seven cases (7/15, 46.67%) had one or two mutations (Table 2).
Table 2.
Comparison of NGS and qPCR results
| NO | NGS | qPCR | Sanger Sequencing |
|---|---|---|---|
| 1 | WT | WT | WT |
| 2 | WT | KRAS G12C | Not sequenced |
| 3 | EGFR E746_T751>VP, 8.54% | EGFR 19del | EGFR E746_T751>VP |
| PIK3CA E545K, 9.60% | PIK3CA E545K | Not sequenced | |
| 4 | EGFR E746_A750delELREA, 35.07% | EGFR 19del | Not sequenced |
| 6 | WT | WT | Not sequenced |
| 7 | WT | WT | Not sequenced |
| 8 | WT | WT | WT |
| 9 | EGFR L858R, 8.26% | EGFR L858R | EGFR L858R |
| EGFR E709K, 3.82% | No Site | Not sequenced | |
| 10 | WT | WT | WT |
| 11 | N/A (inadequate reads) | WT | Not sequenced |
| 12 | WT | WT | WT |
| 13 | EGFR S752_I759delSPKANKEI, 36.36% | WT | EGFR S752_I759delSPKANKEI |
| 15 | EGFR T790M, 65.96% | EGFR T790M | Not sequenced |
| EGFR L858R, 63.89% | EGFR L858R | Not sequenced | |
| 16 | EGFR E746_A750delELREA, 7.57% | EGFR 19del | EGFR E746_A750del |
| EGFR T790M, 3.11% | EGFR T790M | Not detected | |
| 17 | WT | WT | WT |
| 18 | KRAS G13C, 26.95% | No Site | KRAS G13C |
Abbreviations: EGFR, epidermal growth factor receptor; BRAF, v-Raf murine sarcoma viral oncogene homolog B1; KRAS, Kirsten rat sarcoma viral oncogene homolog; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit a; del, deletion; WT, wild type.
A total of 11 mutations were detected: nine EGFR mutations, one KRAS mutation, and one PIK3CA mutation. The nine EGFR mutations were: two leucine-to-arginine substitutions at codon 858 (L858R) in exon 21; two codon 2235_2249 deletions resulting in an in-frame deletion of 5 amino acids from position 746 to 750 (E746_A750del) in exon 19; two T790M with c.2369C>T substitutions in exon 20; one E746_T751>VP with c.2237_2251>TTC deletion in exon 19; one E709K with c.2125G>A substitution in exon 18; and one S752_I759delSPKANKEI with c.2253_2276del24 deletion in exon 19. One KRAS G13C mutation was detected, and it was not accompanied by any EGFR mutation. One PIK3CA E545K mutation was observed, and it coexisted with an EGFR E746_T751>VP mutation. No mutations were detected in BRAF, ALK, PDGFRA, or DDR2.
Comparison of NGS and qPCR results
We next performed qPCR assays to detect mutations in EGFR (45 sites), KRAS (12 sites), PIK3CA (5 sites), and BRAF (1 site). In 12 out of 15 cases, the NGS results were consistent with the qPCR results.
Three mutations were identified by NGS but not by qPCR. Importantly, two of them were not in the detection range of qPCR assays (sample #9 EGFR E709K, and sample #18 KRAS G13C; these are marked as ‘No Site’ in Table 2). Another mutation missed by qPCR was EGFR S752_I759delSPKANKEI (sample #13), and this mutation was in the detection range of qPCR assays. Sanger sequencing confirmed both the KRAS G13C mutation in sample #18 and the EGFR S752_I759delSPKANKEI mutation in sample #13, supporting the NGS result.
One mutation in sample #2, KRAS G12C, was detected by qPCR but not by NGS. Due to insufficient amounts of DNA, we were not able to perform Sanger sequencing for this sample. Taken together, NGS showed high concordance with qPCR, and NGS identified non-hotspot mutations and also provided mutation frequencies and allele sequences.
Molecular testing of histological samples from the same patients using NGS
In our cohort, we had histological specimens for four patients for whom we also had matching cytological samples. The sample #16 was from surgery and others from biopsy. We performed NGS on these histological specimens for all seven genes and compared the results with those obtained from sequencing the cytology specimens of the same patients. Three out of four cases had complete concordance (see Table 3).
Table 3.
Molecular testing of matching tissue samples using NGS
| No. | Cytology specimens | Histological samples | |
|---|---|---|---|
|
|
|||
| NGS | qPCR | NGS | |
| 1 | WT | WT | WT |
| 10 | WT | WT | WT |
| 15 | EGFR p.T790M, 65.96% | EGFR T790M | EGFR p.T790M, 48% |
| EGFR p.L858R, 63.89% | EGFR L858R | EGFR p.L858R, 48% | |
| 16 | EGFR p.E746_A750delELREA, 7.57% | EGFR 19del | EGFR, p.Glu746_Ala750del, 13.6% |
| EGFR p.T790M, 3.11% | EGFR .T790M | ||
The one exception was case #16, in which a deletion in EGFR exon 19 was detected in both cytology and histological samples, but the EGFR T790M mutation was only detected in the cytology sample. The discordance between cytology and histological specimens in this case may be due to tumor heterogeneity.
Clinical observations of DNA Quality Index from 211 consecutive samples
Pleural fluid can be obtained repeatedly and less invasively than biopsy tissues or FNA samples, but it has not been widely used for cancer molecular testing. Of the seven pleural fluid samples that were successfully sequenced by NGS, we detected mutations in two samples (#9 and #16). We noticed that the pleural fluid samples generally had significantly higher DNA concentrations compared to FNA samples (Table 4), and better DNA quality as well (data not shown).
Table 4.
Comparison between FNA and pleural fluid samples
| Sample types | FNA | Pleural fluid |
|---|---|---|
| Total Cases | 10 | 8 |
| Cancer Cellularity (average/median), % | 30~>90 (70/80) | 1~>90 (23.29/30) |
| DNA concentration (average/median), ng/ìL | 0.01~26.89 (7.74/2.24) | 3.93~115.98 (51.91/47.41) |
| Cases for successful NGS (%) | 8 (80) | 8 (100) |
| Cases with gene mutations (%) | 6 (75) | 2 (25) |
To further explore the applicability of using pleural fluid samples as the source for NGS, we systemically evaluated the quality of the DNA extracted from FFPE cell blocks of FNA and pleural fluid samples by employing the DNA Quality Index (DQI) generated by a real-time fluorescent PCR-based assay. The main goal was to compare the DNA quality of FFPE cell blocks of cytology samples with the widely used FFPE tissue blocks. We also included freshly centrifuged plural fluid samples for comparison. A total of 211 consecutive surplus samples were examined (Figure 2), of which 130 were standard FFPE tissue blocks; 27 were FFPE cell blocks of FNA samples; 29 were fresh cells from pleural fluid specimens obtained through centrifugation (pleural fluid fresh); and 25 were FFPE cell blocks of pleural fluid samples. Our results showed that DNA from FFPE cell blocks of pleural fluid samples had a significantly higher DQI than DNA from FFPE cell blocks of FNA samples (P=0.006) and was similar in quality to that from FFPE tissue blocks (P=0.109). In contrast, DNA from FFPE cell blocks of FNA samples had a significantly lower DQI than DNA from FFPE tissue blocks (P=0.049) or from FFPE cell blocks of pleural fluid samples (P=0.006). These results suggest that FFPE cell blocks of pleural fluid samples could be potentially used as starting materials for molecular diagnostic assays when tissue samples are not available.
Figure 2.
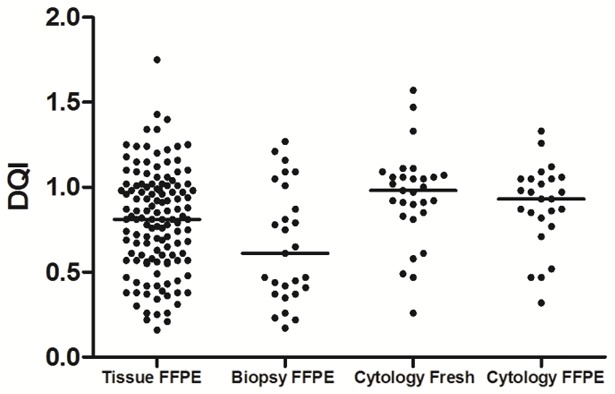
Clinical observation of DNA quality index (DQI) in 211 consecutive surplus samples. The DNA quality of 211 consecutive surplus specimens-including 130 FFPE tissue blocks (FFPE Tissue), 27 FFPE cell blocks of FNA samples (FFPE FNA), 29 freshly centrifuged pleural fluid samples (fresh pleural fluid), and 25 FFPE cell blocks of pleural fluid (FFPE pleural fluid)-was evaluated. The DNA Quality Index and amplifiable DNA concentrations were determined using a DNA Quality Index Kit (Beijing ACCB Biotech Ltd., Beijing, China) according to the manufacturer’s instructions.
Freshly centrifuged plural fluid samples produced DNA of better quality compared to FFPE tissue blocks (P=0.017) and FFPE cell blocks of FNA samples (P=0.004), yet there was no significant difference compared to FFPE cell blocks of pleural fluid samples (P=0.459). This result suggests fresh pleural fluid samples may serve as an alternative option for molecular diagnostics.
Discussion
For various malignancies, molecular testing is important for diagnosis, prognosis, and for guiding targeted therapy. For some patients, cytology specimens may be the only materials available for molecular testing. This is especially true for patients at advanced stages (e.g., stage IIIB or IV), as surgical treatments are not usually recommended for these patients [7]. Hence, it is essential to select a highly efficient method for molecular testing using these small amounts of material. In this study, we evaluated NGS for molecular testing using cytology specimens (FNA and pleural effusion specimens) from lung adenocarcinoma patients. NGS showed several advantages over conventional methods such as qPCR (discussed below). Furthermore, we evaluated histological samples using NGS in four cases and found high concordance between NGS results obtained from cytology samples and from matching histological samples. Our results showed that NGS could be successfully performed on DNA extracted from the cell blocks of FNA and pleural fluid (i.e., cytology) specimens. This approach is particularly important when cytology specimens are the only available samples for molecular testing.
Compared to qPCR, we found NGS using the NextDaySeq Lung panel had several advantages. First, NGS was able to provide accurate variant information. For example, qPCR found a deletion mutation in exon 19 of EGFR in samples #3, #4, and #16, but failed to determine the exact type of mutation due to its multiplexed primer design. NGS, on the other hand, found the exact mutations to be E746_T751>VP and E746_A750delELREA. Second, NGS provided the allele frequencies of identified mutations, which qPCR could not provide. Third, NGS detected two non-hotspot mutations (which were not covered in the qPCR analysis), one in sample #9 (EGFR E709K) and the other in sample #18 (KRAS G13C). Some non-hotspot mutations in EGFR have been shown to affect the efficacy of EGFR tyrosine kinase inhibitors (19, 20). Therefore, NGS can provide important information for clinical reference and research.
To assess how well NGS performed on cytological samples (FNA and pleural fluid samples), we conducted NGS on histological specimens from the same patients. Although matched cytological and histological specimens were available in only four out of 18 cases, NGS results showed high concordance between the specimens, further supporting NGS of cytological specimens as a reliable method of molecular profiling. There was just one disparity between the cytological and histological samples’ NGS results: a deletion in EGFR exon 19 was detected in both cytological and histological samples, but the specific EGFR T790M mutation was only detected in the cytological sample. A recent study has shown that it is common to find cell heterogeneity in different solid tumors, including lung cancer [16]. Because of genetic heterogeneity, a mutation can be present in a certain cell clone but not in others, and the same gene can show different mutations in different clones.
Approximately 15% of lung cancer patients have pleural effusion at the time of initial diagnosis, and 50% of patients develop it later in the course of the disease [21]. Pleural fluid can be obtained repeatedly and less invasively than FNA samples, but it generally has lower neoplastic cellularity than specimens obtained by FNA due to the abundant benign cells in the background [22]. Using NGS, we detected EGFR mutations in two out of five pleural fluid samples that had >20% cellularity, suggesting that pleural fluid, when combined with tumor cellularity analysis, can be used for molecular profiling using NGS. Our study warrants further investigation into the clinical utility of molecular profiling using NGS on pleural fluid specimens.
In many reports, the cytological samples used for molecular analyses are prepared as Diff-Quik smears, ThinPrep slides, or fresh specimens [23]. According to the College of American Pathologists (CAP) guidelines, DNA extraction from cell blocks is more convenient and allows tumor content to be accurately assessed [24]. Cell block specimens also have the advantages of direct tumor cellularity evaluation and sample preservation. Our DQI analysis results showed that DNA from FFPE cell blocks of pleural fluid was comparable in quality to DNA obtained from FFPE tissue blocks, while FFPE cell blocks of FNA had a significantly lower DQI than both FFPE tissue blocks and FFPE cell blocks of pleural fluid. One particularly interesting finding from our DQI analysis was that DNA from freshly centrifuged pleural fluid had a similar quality as DNA from FFPE tissue blocks or FFPE cell blocks of pleural fluid. These findings suggest that pleural fluid samples could be the first choice for molecular diagnostic assays when FFPE tissue blocks are not available.
Acknowledgements
Supported by grant from the Key Research and Development Project of the Department of Science and Technology of Sichuan Province (2018SZ0193). We thank Jiaxing ACCB Diagnostics Ltd. for technical support.
Disclosure of conflict of interest
None.
References
- 1.Shao D, Lin Y, Liu J, Wan L, Liu Z, Cheng S, Fei L, Deng R, Wang J, Chen X, Liu L, Gu X, Liang W. A targeted next-generation sequencing method for identifying clinically relevant mutation profiles in lung adenocarcinoma. Sci Rep. 2016;6:22338. doi: 10.1038/srep22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpa A, Sikora K, Fassan M, Rachiglio AM, Cappellesso R, Antonello D, Amato E, Mafficini A, Lambiase M, Esposito C, Bria E, Simonato F, Scardoni M, Turri G, Chilosi M, Tortora G, Fassina A, Normanno N. Molecular typing of lung adenocarcinoma on cytological samples using a multigene next generation sequencing panel. PLoS One. 2013;8:e80478. doi: 10.1371/journal.pone.0080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med. 2016;4:150. doi: 10.21037/atm.2016.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnes HE, Duncavage EJ, Bernadt CT. Targeted next-generation sequencing using fine-needle aspirates from adenocarcinomas of the lung. Cancer Cytopathol. 2014;122:104–113. doi: 10.1002/cncy.21361. [DOI] [PubMed] [Google Scholar]
- 5.de Biase D, Visani M, Malapelle U, Simonato F, Cesari V, Bellevicine C, Pession A, Troncone G, Fassina A, Tallini G. Next-generation sequencing of lung cancer EGFR exons 18-21 allows effective molecular diagnosis of small routine samples (cytology and biopsy) PLoS One. 2013;8:e83607. doi: 10.1371/journal.pone.0083607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu T, Guo H, Zhao H, Wang L, Zhang Z. Next-generation sequencing for molecular diagnosis of lung adenocarcinoma specimens obtained by fine needle aspiration cytology. Sci Rep. 2015;5:11317. doi: 10.1038/srep11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gailey MP, Stence AA, Jensen CS, Ma D. Multiplatform comparison of molecular oncology tests performed on cytology specimens and formalin-fixed, paraffin-embedded tissue. Cancer Cytopathol. 2015;123:30–39. doi: 10.1002/cncy.21476. [DOI] [PubMed] [Google Scholar]
- 8.Kanagal-Shamanna R, Portier BP, Singh RR, Routbort MJ, Aldape KD, Handal BA, Rahimi H, Reddy NG, Barkoh BA, Mishra BM, Paladugu AV, Manekiah JH, Kalhor N, Chowdhuri SR, Staerkel GA, Medeiros LJ, Luthra R, Patel KP. Next-generation sequencing-based multi-gene mutation profiling of solid tumors using fine needle aspiration samples: promises and challenges for routine clinical diagnostics. Mod Pathol. 2014;27:314–327. doi: 10.1038/modpathol.2013.122. [DOI] [PubMed] [Google Scholar]
- 9.Roh MH. The utilization of cytologic fine-needle aspirates of lung cancer for molecular diagnostic testing. J Pathol Transl Med. 2015;49:300–309. doi: 10.4132/jptm.2015.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heon S, Ortiz TM, Joshi VA, Butaney M, Jackman DM, Kwiatkowski DJ, Yeap BY, Jänne PA, Lindeman NI, Johnson BE. The introduction of systematic genomic testing for patients with non-small cell lung cancer. J Thorac Oncol. 2012;7:1767–1774. doi: 10.1097/JTO.0b013e3182745bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inomata M, Hayashi R, Tanaka H, Shimokawa K, Tokui K, Taka C, Okazawa S, Kambara K, Ichikawa T, Yamada T, Miwa T, Kashii T, Matsui S, Tobe K. Elevated levels of plasma lactate dehydrogenase is an unfavorable prognostic factor in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer, receiving treatment with gefitinib or erlotinib. Mol Clin Oncol. 2016;4:774–778. doi: 10.3892/mco.2016.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda M, Okamoto I, Nakagawa K. Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol. 2014;9:200–204. doi: 10.1097/JTO.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 14.Treece AL, Montgomery ND, Patel NM, Civalier C, Dodd LG, Gulley ML, Booker JK, Weck KE. FNA smears as a potential source of DNA for targeted next-generation sequencing of lung adenocarcinomas. Cancer Cytopathol. 2016;124:406–414. doi: 10.1002/cncy.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S, Lieberman D, Morrissette JJ, Baloch ZW, Roth DB, McGrath C. Using “Residual” FNA rinse and body fluid specimens for next-generation sequencing: an institutional experience. Cancer Cytopathol. 2016;124:324–329. doi: 10.1002/cncy.21666. [DOI] [PubMed] [Google Scholar]
- 16.Buttitta F, Felicioni L, Grammastro MD, Filice G, Lorito AD, Malatesta S, Viola P, Centi I, D’Antuono T, Zappacosta R, Rosini S, Cuccurullo F, Marchetti A. Effective assessment of egfr mutation status in bronchoalveolar lavage and pleural fluids by next-generation sequencing. Clin Cancer Res. 2013;19:691–698. doi: 10.1158/1078-0432.CCR-12-1958. [DOI] [PubMed] [Google Scholar]
- 17.Rehm HL, Bale SJ, Bayrak-Toydemir P, Berg JS, Brown KK, Deignan JL, Friez MJ, Funke BH, Hegde MR, Lyon E The Working Group of the American College of Medical Genetics and Genomics Laboratory Quality Assurance. ACMG clinical laboratory standards for next-generation sequencing. Genet Med. 2013;15:733–747. doi: 10.1038/gim.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Mercier M, D’Haene N, De Nève N, Blanchard O, Degand C, Rorive S, Salmon I. Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology. 2015;66:215–224. doi: 10.1111/his.12461. [DOI] [PubMed] [Google Scholar]
- 19.Wu JY, Shih JY. Effectiveness of tyrosine kinase inhibitors on uncommon E709X epidermal growth factor receptor mutations in non-small-cell lung cancer. Onco Targets Ther. 2016;9:6137–6145. doi: 10.2147/OTT.S118071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu CH, Yu LK, Zhan P, Zhang Y. Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. Eur J Med Res. 2014;19:23. doi: 10.1186/2047-783X-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linder J. Lung cancer cytology. Something old, something new. Am J Clin Pathol. 2000;114:169–171. doi: 10.1309/BB5Y-1J1Q-6LUU-YW9Y. [DOI] [PubMed] [Google Scholar]
- 23.Vigliar E, Malapelle U, de Luca C, Bellevicine C, Troncone G. Challenges and opportunities of next-generation sequencing: a cytopathologist’s perspective. Cytopathology. 2015;26:271–283. doi: 10.1111/cyt.12265. [DOI] [PubMed] [Google Scholar]
- 24.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the college of American pathologists, international association for the study of lung cancer, and association for molecular pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]


