Abstract
Exportin-1 (XPO1) is an essential nuclear export receptor that is involved in the pathogenesis of multiple tumors. However, the role of XPO1 in diffuse large B-cell lymphoma (DLBCL) requires clarification. This study aims to detect XPO1 expression in DLBCL and to explore its relationships with clinicopathologic parameters and prognoses. Methods: A total of 131 cases of DLBCL and 30 cases of reactive lymphoid hyperplasia were selected for immunohistochemistry to examine XPO1 expression and analyze the relationships of XPO1 expression with clinicopathologic parameters and prognosis. DLBCL datasets downloaded from The Cancer Genome Atlas (TCGA) were used to analyze the mutations, expressions, and clinical values of XPO1 in DLBCL. Results: XPO1 expression was markedly upregulated in DLBCL compared to the reactive lymphoid hyperplasia group (χ2 = 10.734, P = 0.001). High XPO1 expression was associated with an advanced clinical stage (χ2 = 4.036, P = 0.045) and a risky International Prognostic Index (IPI) score (χ2 = 5.301, P = 0.025). Moreover, high XPO1 expression was associated with a lower overall survival rate compared with low expression (P = 0.043). XPO1 was an independent prognostic factor for DLBCL (risk ratio, RR = 3.772, P = 0.006). Furthermore, XPO1 overexpression in DLBCL was correlated with a high IPI score (P = 0.024) in TCGA datasets. Conclusion: High XPO1 expression in DLBCL was related to an advanced clinical stage, poor IPI score, and poor prognosis. Thus, XPO1 may be useful for condition identification and prognostic assessment.
Keywords: Diffuse large B-cell lymphoma, exportin-1, clinicopathological significance, prognosis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma (NHL) [1-3]. Each DLBCL subtype has different pathological features, biological behaviors, clinical presentations, and prognoses [4,5]. DLBCL is categorized into the germinal center B cell-like (GCB) and non-germinal center B cell-like (non-GCB) subtypes based on immunohistochemistry tests [6-8]. The GCB subtype has a better prognosis [9]. Currently, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is used as the first-line chemotherapy for DLBCL because this regimen improves the prognosis and results in 70-80% complete remission (CR) and 50-60% progression-free survival (PFS) at 3-5 years [10-14]. However, one-third of patients with DLBCL are refractory to the treatment or experience recurrence after treatment [15,16]. Hence, providing treatment for patients who resist the first-line therapy or have early recurrence is a pressing clinical issue. The identification of effective DLBCL markers for diagnosis and prognosis that will lead to accurate diagnostic stratification and individualized treatment is of paramount importance.
Exportin-1 (XPO1), also known as chromosome region maintenance 1 (CRM1), mediates leucine-rich nuclear export signal (NES)-dependent protein transport [17,18]. Current studies have revealed that XPO1 nuclear export pathways are involved in the protein regulation and signal transduction of multiple key molecules, including p21, p27, p53, and epidermal growth factor (EGF) [19,20]. Additionally, XPO1 expression is increased and has been related to an unfavorable prognosis in malignancies, including ovarian cancer [21], multiple myeloma [17], and acute myeloid leukemia [22]. A number of studies have shown that XPO1 can function as a target for oncotherapy [23-26], although studies on its role in DLBCL are limited. The gene chip analyses performed by Kwiecinska [27], Scholtysik [28], and Trifonov [29] indicated that XPO1 was highly expressed in DLBCL tissues. Zhou [30] found that XPO1 was positively expressed in DLBCL. However, comprehensive research on the relationships between XPO1 expression, clinical parameters, and prognosis is lacking.
This study aims to explore the correlation of XPO1 expression in DLBCL with its clinicopathological parameters and prognosis. Additionally, we analyze the biologic significance of XPO1 using TCGA datasets to provide evidence for the clinical diagnosis and treatment of DLBCL.
Materials and methods
Tissue samples
A total of 131 paraffin specimens of primary DLBCL were collected and re-examined as sections. All of the specimens were pathologically diagnosed using the 2008 World Health Organization (WHO) criteria. The age of onset ranged from 18 to 86 (median 54) years. Of the specimens, 43 cases were diagnosed with lymphadenopathy, mainly iun the neck and inguinal lymph nodes, and 88 cases were classified as extranodal lesions, mainly in the stomach, intestines, liver, spleen, and brain; among the latter cases, 20 were primary central nervous system lymphoma. Of the 131 patients, 78 had received chemotherapy. Among these patients, 29 had been treated with CHOP therapy and 5 were in CR while 6 were in partial remission (PR). Additionally, 39 patients had elected to receive R-CHOP treatment (20 were in CR and 9 were in PR), while 10 patients preferred other forms of chemotherapy. Clinical staging was conducted based on the 2008 WHO Ann Arbor criteria, and the performance status of the patients was measured using the Eastern Cooperative Oncology Group (ECOG) and International Prognostic Index (IPI) scores. None of the patients received antineoplastic therapy prior to the biopsy or operation, and the patients had no medical history of immunodeficiency. The follow-up, which lasted between 14 and 65 months, ended on October 15, 2016. The overall survival (OS) and PFS were calculated separately. Thirty cases of reactive hyperplasia of the lymph node were selected as the controls. The study was approved by the Research Ethics Committees of the First Affiliated Hospital of Guangxi Medical University, China.
Reagents
The XPO1 antibody (sc-5595) was purchased from Santa Cruz and used at a 1:100 dilution. The ready-to-use Bcl-2 and Bcl-6 antibodies were acquired from Fuzhou Maixin Biotech. Co., Ltd. CD20, MUM1, CD10, and Ki67 were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
Immunohistochemistry
Paraffin-embedded tissues were sliced into 4-μm sections. Then, the slides were baked in an oven for 6 hours. The slides were subjected to dewaxing and gradient hydration with ethanol. An ethylenediamine tetra acetic acid buffer solution was applied to recover antigens, according to the manufacturer’s instructions, and 3% H2O2 was used as a block. The primary antibodies were added first. The sections with the XPO1 antibody were incubated at 4°C overnight. Then, the secondary antibody was added after a 30-minute rewarming period. The sections with the ready-to-use primary antibodies were incubated at 37°C for 1 hour, after which the secondary antibodies were added, and the slides were incubated for 30 minutes and washed three times with phosphate-buffered saline (PBS). Next, the slides were incubated with 3,3’-diaminobenzidine for visualization for 2-5 minutes. Re-staining was performed with hematoxylin, and dehydration was conducted for transparency. Finally, neutral balsam was used as the mounting medium. Positive tissues were considered as the positive control group, and PBS was used in place of the primary antibody as the normal control group [31,32].
Immunohistochemistry assessment
XPO1 expression was located in the nucleus and partly in the cytoplasm. Bcl-2, CD20, and CD10 were detected in the cytomembrane, and Bcl-6, MUM1, and Ki67 were found in the nucleus. The overall scoring system was based on the staining intensity of the tumor cells and the percentage of positive cells [33]. The stain color was compared against the background color in the same section. The scoring criteria for the staining intensity were as follows: 0 for colorless, 1 for light yellow, 2 for yellowish-brown, and 3 for dark brown. The scoring system for the percentages of positive cells was as follows: 0 for ≤ 5%, 1 for 6%-25%, 2 for 26%-50%, 3 for 51%-75%, and 4 for > 75%. The overall score was achieved by multiplying the intensity score by the percentage score: 0-4 was considered negative (-), 5-8 positive (+), and 9-12 strongly positive (++). “Positive” and “strongly positive” were classified as high expression. Additionally, a sample was considered positive when more than 25% of the cells stained positive for Bcl-2, CD20, CD10, Bcl-6, and MUM1 and more than 70% of the cells stained positive for Ki67.
Analysis of TCGA datasets
Mutations in XPO1 in DLBCL (TCGA, provisional) were analyzed with cBioPortal (www.cbioportal.org) [34,35], which included amplification, mRNA upregulation, and missense mutations. OncoPrint in cBioPortal was employed to draw the XPO1 mutation diagram for the 48 DLBCL cases. Additionally, plot was applied to generate several pictures of the clinicopathological parameters (Neoplasm American Joint Committee on Cancer Clinical Group Stage), OS, and DFS. The clinical data for XPO1 expression in DLBCL downloaded from TCGA were used to analyze the correlations between XPO1 and the clinical parameters and prognosis. To obtain a better understanding of the relationships between XPO1 and other genes, the network of XPO1 and related genes was downloaded from cBioPortal.
Statistical analysis
SPSS 22.0 (IBM Corporation) was used for statistical analysis. Quantitative data were represented as the mean ± standard deviation, and categorical data were analyzed with the χ2 test or Spearman’s rank correlation. The Kaplan-Meier method was applied for survival analysis, and comparisons between curves were tested with the log-rank test. A Cox proportional hazards model was adopted for analysis of the effects of multiple factors on the prognosis. Statistical significance was set at P < 0.05.
Results
Morphology of the tissue sections
We observed HE-stained DLBCL and reactive lymphoid hyperplasia tissue sections using microscopy. The observation of typical DLBCL morphology showed that the tissues in or outside of the lymph nodes were replaced by diffuse cells that contained nuclei that were approximately twice as large as the nuclei of small lymphocytes. The large cells had a rich endochylema, empty nucleus, and obvious nucleolus (Figure 1A). The reactive lymph node hyperplasia sections revealed the partial existence of a normal structure with an increased number of lymphoid follicles, larger size, and expanded germinal center; additionally, the paracortical area was broadened, and the vessels were multiplied (Figure 1B).
Figure 1.
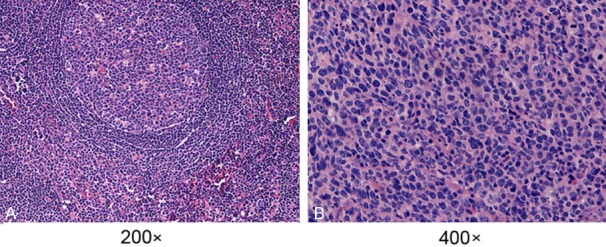
Morphology of H&E-stained DLBCL and reactive lymph node hyperplasia tissue sections. A. DLBCL tissues; B. reactive lymph node hyperplasia tissue. Annotation: DLBCL, diffuse large B-cell lymphoma; H&E, hematoxylin-eosin.
Immunohistochemical staining results
Positive XPO1 expression in the DLBCL sections was mostly detected in the nuclei of the tumor cells (Figure 2A). Additionally, some staining was detected in the cytoplasm, with a positive percentage of 56.1% (74/131). XPO1 had extremely low or no expression in the reactive lymph node hyperplasia sections (Figure 2B). The majority of the expression was detected in the germinal center, while a small amount was seen in the medullary cord and medullary sinus. Positive expression was located in the cytoplasm and nucleus in 23.3% (7/30) of the sections, which was significantly lower than the positive staining rate detected in the DLBCL group (χ2 = 10.734, P = 0.001). CD20 expression was positive in all 131 cases, and Bcl-2 and Ki67 expression was positive in 80.2% (105/131) and 63.4% (83/131) of the cases, respectively. The staining results for CD20, Bcl-2, Bcl-6, MUM1, CD10, and Ki67 are shown in Figure 3.
Figure 2.
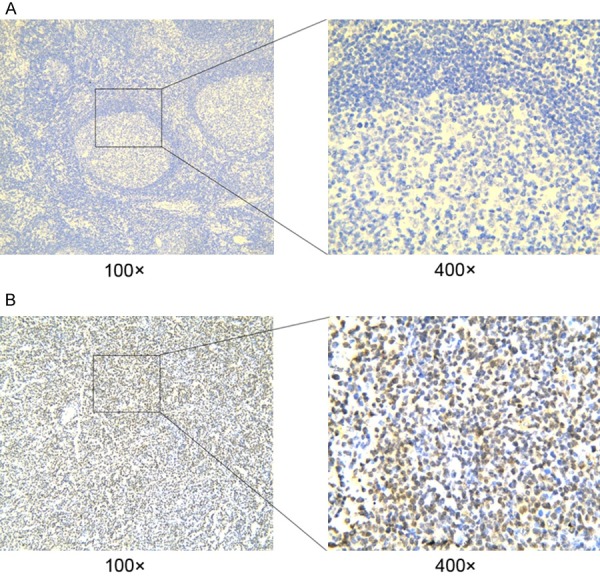
Detection of XPO1 protein expression by immunohistochemistry in DLBCL and reactive lymph node hyperplasia tissues. A: DLBCL tissues; B: reactive lymph node hyperplasia tissues. Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1.
Figure 3.
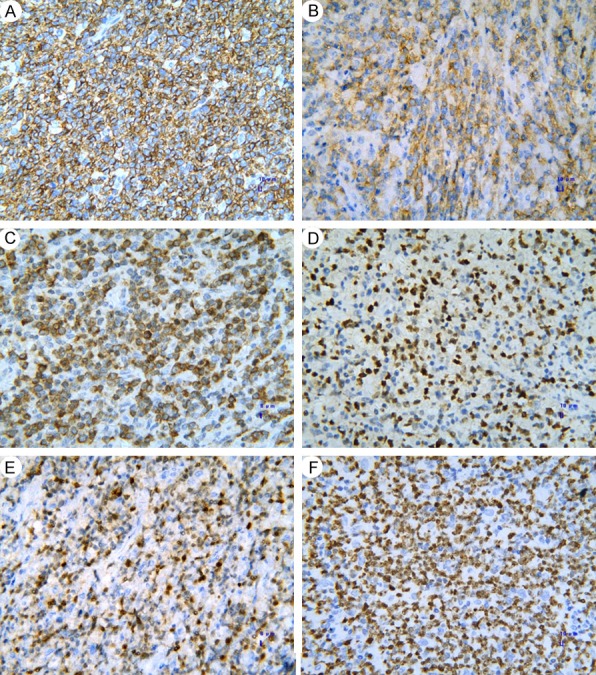
Detection of CD20, CD10, Bcl-2, Bcl-6, MUM1, and Ki67 protein expression in DLBCL tissues by immunohistochemistry. A. CD20; B. CD10; C. Bcl-2; D. Bcl-6; E. MUM1; F. Ki67 (400×). Annotation: DLBCL, diffuse large B-cell lymphoma.
Relationships between XPO1 expression and clinicopathological parameters in DLBCL patients
The positive percentages of XPO1 expression in the GCB subtype (CD10+; CD10-/Bcl-6+/MUM1-) and the non-GCB subtype (CD10-/Bcl-6+/MUM1+; CD10-/Bcl-6-/MUM1+; CD10-/Bcl-6-/MUM1-) were 47.4% (18/38) and 60.3% (56/93), respectively. The increased XPO1 expression was associated with an advanced clinical stage (χ2 = 4.036, P = 0.045) and a high IPI score (χ2 = 5.301, P = 0.025) (Table 1). However, high XPO1 expression showed no relationship with the age, gender, extranodal involvement, B symptoms, subtypes, ECOG scores, hemoglobin (Hb), lactate dehydrogenase (LDH), Bcl-2, or Ki67 parameters (P > 0.05).
Table 1.
Relationships between XPO1 expression and clinicopathologic parameters in DLBCL patients
| Clinicopathologic parameters | n | XPO1 | χ2 | P | r | P | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| + | - | ||||||
| Gender | |||||||
| Male | 77 | 41 | 36 | 0.799 | 0.371 | -0.078 | 0.375 |
| Female | 54 | 33 | 21 | ||||
| Age | |||||||
| > 60 | 49 | 29 | 20 | 0.231 | 0.631 | 0.042 | 0.634 |
| ≤ 60 | 82 | 45 | 37 | ||||
| Extranodal sites | |||||||
| < 2 | 92 | 50 | 42 | 0.576 | 0.448 | -0.060 | 0.452 |
| ≥ 2 | 39 | 24 | 15 | ||||
| Clinical stage | |||||||
| III-IV | 59 | 39 | 20 | 4.036 | 0.045* | 0.176 | 0.045* |
| I-II | 72 | 35 | 37 | ||||
| LDH level | |||||||
| High | 51 | 26 | 25 | 0.382 | 0.537 | -0.062 | 0.541 |
| Normal | 49 | 28 | 21 | ||||
| ECOG PS | |||||||
| < 2 | 98 | 56 | 42 | 0.158 | 0.691 | 0.035 | 0.694 |
| ≥ 2 | 32 | 17 | 15 | ||||
| IPI score | |||||||
| 3-5 | 47 | 33 | 14 | 5.031 | 0.025* | 0.196 | 0.025* |
| 0-2 | 84 | 42 | 42 | ||||
| B symptoms | |||||||
| Yes | 29 | 15 | 14 | 0.297 | 0.585 | -0.048 | 0.589 |
| No | 101 | 58 | 43 | ||||
| Hgb | |||||||
| Low | 64 | 38 | 26 | 0.378 | 0.539 | 0.055 | 0.542 |
| Normal | 63 | 34 | 29 | ||||
| Chemotherapy | |||||||
| CHOP | 29 | 16 | 13 | 0.101 | 0.751 | 0.039 | 0.755 |
| R-CHOP | 39 | 20 | 19 | ||||
| Treatment responses | |||||||
| CR+PR | 44 | 24 | 20 | 0.007 | 0.934 | -0.010 | 0.935 |
| SD+PD | 27 | 15 | 12 | ||||
| Subtype | |||||||
| GCB | 38 | 18 | 20 | 1.811 | 0.178 | -0.118 | 0.181 |
| Non-GCB | 93 | 56 | 37 | ||||
| Bcl-2 | |||||||
| + | 105 | 61 | 44 | 0.556 | 0.456 | 0.065 | 0.460 |
| - | 26 | 13 | 13 | ||||
| Ki67 | |||||||
| + | 83 | 47 | 36 | 0.002 | 0.967 | 0.004 | 0.967 |
| - | 48 | 27 | 21 | ||||
Annotation: XPO1, exportin-1; DLBCL, diffuse large B-cell lymphoma; LDH, lactate dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; Hgb, hemoglobin; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP, rituximab, cyclophosphamide, daunorubicin, vincristine, prednisone; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; GCB, germinal center B cell-like; non-GCB, non-germinal center B cell-like;
P < 0.05.
Follow-up results and survival analysis
Of the 131 patients with DLBCL, 58 were deceased, resulting in a mortality rate of 46.8% (7 were lost to follow-up). The three-year OS rate was 54.9%, and the 5-year OS rate was 29.4%, with a median survival time of 41.0 months (range 37.8-44.2 months). The univariate survival analysis revealed that higher XPO1 expression was correlated with a worse prognosis (OS P = 0.043, PFS P = 0.011; Figure 4A, 4B). Moreover, there was a close link between a worse prognosis and an elderly age, non-GCB subtype, high LDH level, Bcl-2 expression, Ann Arbor stages III-IV, and high IPI score (Table 2). The Cox regression analysis indicated that the XPO1 protein expression and LDH levels were independent factors that influenced the OS of DLBCL patients and that the XPO1 and LDH levels, the clinical stage, and R-CHOP were independent factors that influenced the PFS of DLBCL patients. The risk ratios (RRs) of high XPO1 and LDH expression for OS were 3.772 (P = 0.006) and 10.375 (P < 0.001), respectively. The RRs of XPO1 and LDH expression, Ann Arbor stages III-IV, and R-CHOP treatment for PFS were 3.595 (P = 0.002), 5.474 (P < 0.001), 2.331 (P = 0.027), and 0.359 (P = 0.007), respectively. After analyzing the prognoses of the 78 patients who received chemotherapy for DLBCL, we found that high XPO1 expression was associated with a poor prognosis (OS P = 0.034, PFS P = 0.020; Figure 4C, 4D). Additionally, patients of the non-GCB subtype who had a high LDH level, high Bcl-2 expression, Ann Arbor stages III-IV, or a high IPI score had an undesirable prognosis (Table 3). The multiple factor analysis of the OS of the 78 patients who received chemotherapy suggested that the XPO1 (RR = 3.772, P = 0.006) and LDH (RR = 10.375, P < 0.001) expression levels were independent factors for the DLBCL prognosis, which was similar to the overall analysis results for the 131 patients with DLBCL.
Figure 4.
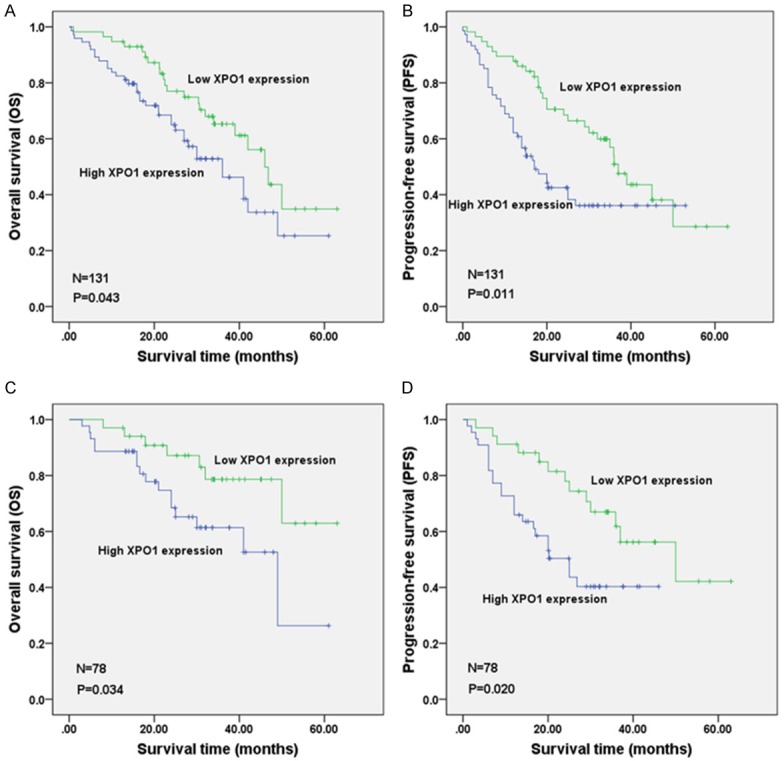
Survival comparison of DLBCL patients with low and high XPO1 expression levels. A: overall survival (OS) of 131 DLBCL patients; B: progression-free survival (PFS) of 131 DLBCL patients; C: OS of 78 DLBCL patients with chemotherapy; D: PFS of 78 DLBCL patients undergoing chemotherapy. Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1.
Table 2.
Univariate prognostic analysis of clinicopathologic parameters in 131 DLBCL patients
| Risk factors | n | 3-year OS (%) | 5-year OS (%) | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 77 | 59.5 | 27.6 | 0.185 |
| Female | 54 | 47.7 | 32.5 | |
| Age | ||||
| > 60 | 49 | 42.7 | 30.5 | 0.036* |
| ≤ 60 | 82 | 68.0 | 27.9 | |
| Extranodal sites | ||||
| < 2 | 92 | 58.8 | 35.1 | 0.160 |
| ≥ 2 | 39 | 45.9 | 15.1 | |
| Clinical stage | ||||
| III-IV | 59 | 41.2 | 27.4 | 0.035* |
| I-II | 72 | 65.5 | 30.6 | |
| LDH level | ||||
| High | 51 | 39.2 | 17.6 | < 0.001* |
| Normal | 49 | 84.8 | 63.6 | |
| ECOG PS | ||||
| < 2 | 98 | 55.3 | 33.1 | 0.526 |
| ≥ 2 | 32 | 51.9 | 26.0 | |
| IPI score | ||||
| 3-5 | 47 | 31.4 | 19.6 | < 0.001* |
| 0-2 | 84 | 68.6 | 34.8 | |
| B symptoms | ||||
| Yes | 29 | 45.6 | 0.0 | 0.171 |
| No | 101 | 56.7 | 36.2 | |
| Hgb | ||||
| Low | 64 | 47.4 | 8.0 | 0.080 |
| Normal | 63 | 58.1 | 48.2 | |
| Subtype | ||||
| GCB | 38 | 70.1 | 52.7 | 0.031* |
| Non-GCB | 93 | 48.4 | 15.7 | |
| Treatment | ||||
| No | 53 | 37.6 | 13.7 | < 0.001* |
| Yes | 78 | 69.1 | 46.2 | |
| Chemotherapy | ||||
| R-CHOP | 39 | 74.0 | 74.0 | 0.101 |
| CHOP + other | 39 | 64.5 | 17.9 | |
| XPO1 | ||||
| + | 74 | 46.2 | 25.3 | 0.043* |
| - | 57 | 65.3 | 34.9 | |
| Bcl-2 | ||||
| + | 105 | 53.3 | 18.8 | 0.028* |
| - | 26 | 61.2 | 61.2 | |
| Ki67 | ||||
| + | 83 | 57.7 | 32.5 | 0.419 |
| - | 48 | 50.4 | 25.6 |
Annotation: DLBCL, diffuse large B-cell lymphoma; OS, overall survival; LDH, lactate dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; Hgb, hemoglobin; GCB, germinal center B cell-like; non-GCB, non-germinal center B cell-like; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP, rituximab, cyclophosphamide, daunorubicin, vincristine, prednisone; other chemotherapy including methotrexate or temozolomide;
P < 0.05.
Table 3.
Univariate prognostic analysis of clinicopathologic parameters in 78 DLBCL patients with chemotherapy
| Risk factors | n | 3-year OS (%) | 5-year OS (%) | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 44 | 72.1 | 38.4 | 0.625 |
| Female | 34 | 65.4 | 65.4 | |
| Age | ||||
| > 60 | 23 | 65.6 | 65.6 | 0.969 |
| ≤ 60 | 55 | 70.8 | 38.9 | |
| Extranodal sites | ||||
| < 2 | 53 | 58.4 | 58.4 | 0.263 |
| ≥ 2 | 25 | 52.1 | 26.0 | |
| Clinical stage | ||||
| III-IV | 41 | 57.1 | 37.5 | 0.021* |
| I-II | 37 | 83.5 | 55.7 | |
| LDH level | ||||
| High | 35 | 43.2 | 21.6 | < 0.001* |
| Normal | 40 | 90.4 | 90.4 | |
| ECOG PS | ||||
| < 2 | 58 | 70.0 | 63.0 | 0.491 |
| ≥ 2 | 19 | 65.2 | 39.1 | |
| IPI score | ||||
| 3-5 | 30 | 45.3 | 27.2 | 0.001* |
| 0-2 | 48 | 86.0 | 57.3 | |
| B symptoms | ||||
| Yes | 18 | 65.8 | 0.0 | 0.520 |
| No | 59 | 68.8 | 55.0 | |
| Hgb | ||||
| Low | 34 | 62.8 | 16.7 | 0.234 |
| Normal | 41 | 71.6 | 71.6 | |
| Subtype | ||||
| GCB | 22 | 78.7 | 78.7 | 0.044* |
| Non-GCB | 56 | 65.8 | 19.2 | |
| Chemotherapy | ||||
| R-CHOP | 39 | 74.0 | 74.0 | 0.101 |
| CHOP + other | 39 | 64.5 | 17.9 | |
| XPO1 | ||||
| + | 44 | 61.4 | 26.3 | 0.034* |
| - | 34 | 78.6 | 62.9 | |
| Bcl-2 | ||||
| + | 61 | 66.7 | 30.0 | 0.076 |
| - | 17 | 77.1 | 77.1 | |
| Ki67 | ||||
| + | 49 | 70.5 | 47.0 | 0.700 |
| - | 29 | 67.0 | 55.9 |
Annotation: DLBCL, diffuse large B-cell lymphoma; OS, overall survival; LDH, lactate dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; Hgb, hemoglobin; GCB, germinal center B cell-like; non-GCB, non-germinal center B cell-like; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP, rituximab, cyclophosphamide, daunorubicin, vincristine, prednisone; other chemotherapy including methotrexate or temozolomide;
P < 0.05.
TCGA data analysis
According to OncoPrint, the mutation rate of XPO1 was 19% in the 48 DLBCL cases (TCGA, provisional) (Figure 5). No significant relationship was noted between XPO1 mRNA expression and the clinical stage (Figure 6). There was also no significant relation found between the mutations and DFS (Figure 7A) or OS (Figure 7B) (P > 0.05). In TCGA, complete case information was obtained for the 47 DLBCL cases, which were divided into high and low expression groups using the median expression level (Table 4). High XPO1 expression levels were associated with an elderly age (χ2 = 7.982, P = 0.005) and risky IPI score (P = 0.024), whereas no correlation was found between XPO1 and gender, extranodal involvement, clinical stage, ECOG, or the therapeutic model (P > 0.05). The single factor analysis revealed that increased XPO1 expression was most likely a sign of a poor prognosis, but no statistical significance was observed (OS P = 0.159, DFS P = 0.139, Figure 8). No independent factors that might affect the DLBCL prognosis were found in the Cox regression analysis. The gene network illustrated the interaction between XPO1 and other genes in DLBCL (Figure 9). Moreover, the network showed the molecular targeting drug for XPO1 (Compound 7d-cis), which has not been approved by the FDA.
Figure 5.
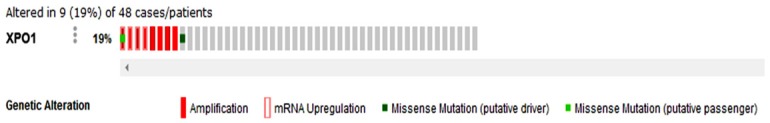
Genetic alterations of XPO1 in DLBCL (TCGA, provisional). Genetic alterations in XPO1, including amplification, mRNA upregulation, and missense mutations, were identified in DLBCL using OncoPrint from cBioPortal (www.cbioportal.org). Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1; TCGA, The Cancer Genome Atlas.
Figure 6.
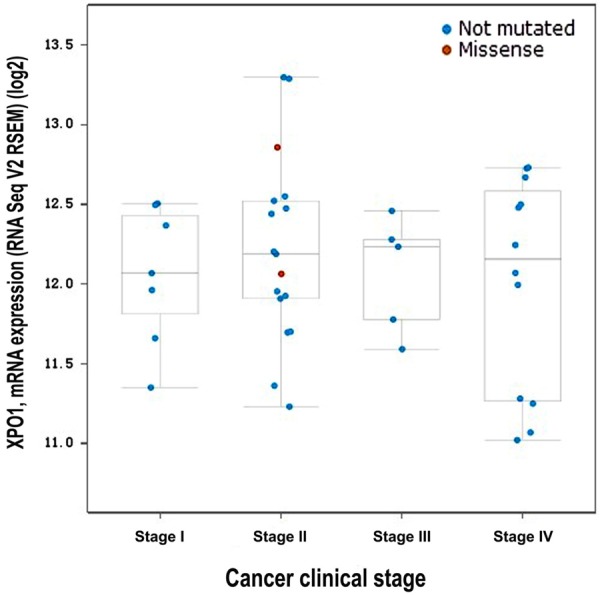
Connection between the XPO1 mRNA level and the clinical stage in DLBCL (TCGA, provisional). The log2 mRNA expression levels from RNA Seq V2 RSEM were downloaded from TCGA data. The Plot figure was drawn using cBioPortal (www.cbioportal.org) to show the connection between mRNA expression and the clinical stage (P > 0.05). Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1; TCGA, The Cancer Genome Atlas.
Figure 7.
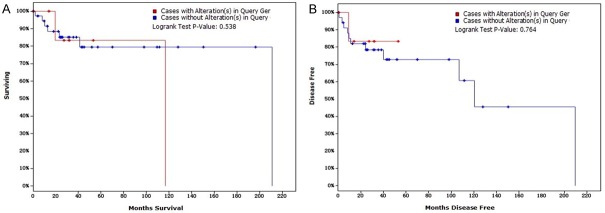
Relationship between XPO1 alterations and survival in DLBCL (TCGA, provisional). A. Overall survival (OS): two out of 9 cases with alterations resulted in death, and the median survival time was 116.72 months. Seven out of 38 cases without alterations resulted in death, and the median survival time was 211.07 months; B. Disease-free survival (DFS): one of the 8 cases with alterations relapsed, and the median time of DFS was 120.53 months, whereas 11 of the 35 cases without alterations relapsed. Survival was analyzed using the Kaplan-Meier estimate provided by cBioPortal (www.cbioportal.org). Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1; TCGA, The Cancer Genome Atlas.
Table 4.
Relationships between XPO1 expression and clinicopathological parameters in DLBCL patients in TCGA datasets
| Clinicopathological parameters | n | XPO1 | χ2 | P | r | P | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| + | - | ||||||
| Gender | |||||||
| Male | 21 | 10 | 11 | 0.180 | 0.671 | -0.062 | 0.679 |
| Female | 26 | 14 | 12 | ||||
| Age | |||||||
| > 60 | 20 | 15 | 5 | 7.982 | 0.005* | 0.412 | 0.004* |
| ≤ 60 | 27 | 9 | 18 | ||||
| Extranodal sites | |||||||
| < 2 | 25 | 13 | 12 | 0.239 | 0.625 | -0.139 | 0.413 |
| ≥ 2 | 12 | 8 | 4 | ||||
| Clinical stage | |||||||
| III-IV | 17 | 9 | 8 | 0.034 | 0.853 | 0.029 | 0.857 |
| I-II | 24 | 12 | 12 | ||||
| ECOG PS | |||||||
| < 2 | 28 | 12 | 16 | 0.031 | 0.859 | 0.141 | 0.448 |
| ≥ 2 | 3 | 2 | 1 | ||||
| IPI score | |||||||
| 3-5 | 6 | 6 | 0 | - | 0.024* | 0.452 | 0.016* |
| 0-2 | 22 | 10 | 12 | ||||
| Treatment | |||||||
| Chemotherapy | 22 | 11 | 11 | - | 1.000 | 0.204 | 0.350 |
| Chemotherapy + radiotherapy | 1 | 0 | 1 | ||||
| Treatment responses | |||||||
| CR+PR | 21 | 11 | 10 | 0.082 | 0.775 | -0.167 | 0.425 |
| SD+PD | 4 | 3 | 1 | ||||
Annotation: XPO1, exportin-1; DLBCL, diffuse large B-cell lymphoma; TCGA, The Cancer Genome Atlas; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease;
P < 0.05.
Figure 8.
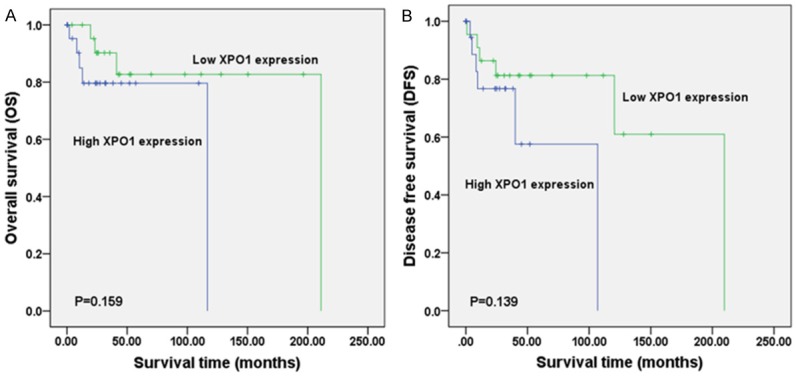
Survival comparison between DLBCL patients from TCGA datasets with low and high XPO1 expression levels (TCGA, provisional). A: overall survival (OS); B: disease-free survival (DFS). Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1; TCGA, The Cancer Genome Atlas.
Figure 9.
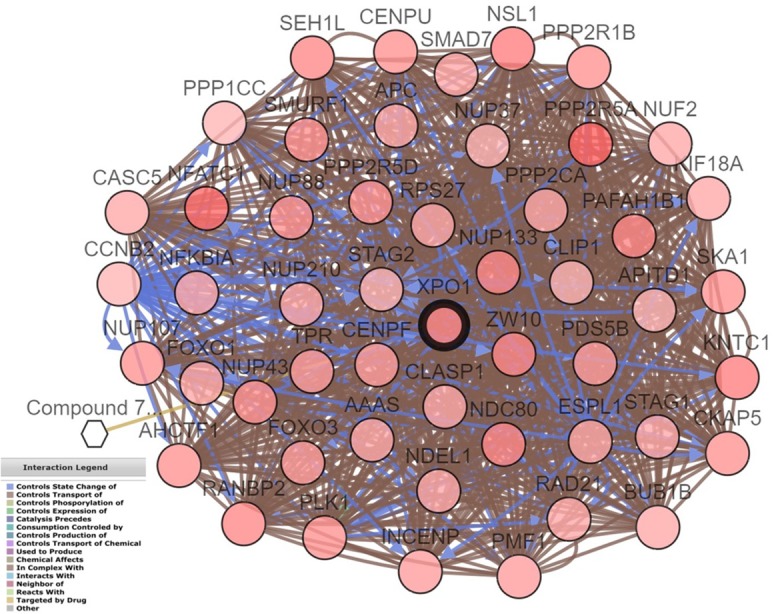
Drugs targeting specific gene networks related to XPO1 in DLBCL (TCGA, provisional). The gene network for XPO1 in DLBCL was drawn using cBioPortal (www. cbioportal.org). Circles indicate genes, and hexagons represent drugs. XPO1 is targeted by Compound 7d-cis, which has not been approved by the FDA. Annotation: DLBCL, diffuse large B-cell lymphoma; XPO1, exportin-1; TCGA, The Cancer Genome Atlas; FAD, Food and Drug Administration.
Discussion
Recent studies on biomarkers of DLBCL and its prognosis have attracted attention. Dekker [36] found that FOXP1 affected the growth of DLBCL cells and that high FOXP1 expression was associated with the prognosis. Lu [37] discovered that high Myc and Bcl-2 expression and mutations were associated with a negative prognosis. Additionally, metadherin (MTDH) prohibited proliferation of tumor cells and strengthened chemo-sensitivity to doxorubicin in DLBCL [38]. The NF-κB signaling pathway inhibited the apoptosis of DLBCL cells, thereby reducing the chemo-sensitivity of the tumor cells [39]. Due to the significant heterogeneity of DLBCL and the undesirable clinical treatment, identifying key biological factors and seeking individualized treatment strategies are urgent issues.
Current studies have demonstrated that XPO1 is involved in the oncogenesis and progression of multiple tumors. High XPO1 expression was observed in kidney cancer, and inhibition of XPO1 resulted in the death of cancer cells and upregulation of p53 and p21 [40]. An XPO1 inhibitor had a suppressing effect on the proliferation of prostate cancer cells and induced apoptosis; the inhibitor also prevented the nuclear export of FOXO and p53 [41]. In addition, XPO1 prevented the apoptosis of tumor cells in acute leukemia [22,42], and its overexpression predicted a negative prognosis. Because XPO1 functions as a prognostic marker for various tumors, the development of inhibitors of this protein has been increasing; in particular, pre-clinical studies on mantle cell lymphoma showed great potential value for XPO1 research [43]. Inhibitors of XPO1 prohibited the proliferation of NHL cells and induced their apoptosis by down-regulating anti-apoptotic proteins, such as Survivin and NF-κB [42,44]. However, research on the clinical significance and mechanism of XPO1 in DLBCL is lacking.
To explore the clinical role of XPO1 in DLBCL, we applied immunohistochemistry to detect XPO1 expression. We found that XPO1 was overexpressed in DLBCL tissue, which was consistent with the results of Zhou’s study [30]. Additionally, XPO1 overexpression was related to an advanced clinical stage and a high IPI score. Currently, IPI is the most frequently used index for assessing the prognosis of DLBCL patients. The index is critical for the determination of the prognosis and clinical therapeutics, although it has failed to completely assess patients’ statuses [11]. Univariate survival analysis indicated that high XPO1 expression was a poor prognostic factor for DLBCL because the OS and PFS were longer in the low XPO1 expression group. In the chemotherapy group, XPO1 exerted more remarkable effects on the prognosis, possibly because the patients had quicker progression and higher mortality without chemotherapy; thus, XPO1 expression had little impact on the prognosis. Conversely, analysis of both groups (the 131 DLBCL patients and the 78 patients receiving chemotherapy) revealed that a poor prognosis was associated with a non-GCB subtype, high LDH level, high Bcl-2 expression, Ann Arbor stages III-IV, and a risky IPI score. Similar results were found in previous studies [9,11], validating our research. The multivariate survival analysis revealed that XPO1 was an independent prognostic factor for DLBCL and has the potential of becoming a clinical prognostic indicator for DLBCL. In the TCGA datasets, high XPO1 expression in DLBCL was connected with an elderly age and a dangerous IPI score, while high XPO1 expression was a possible sign of a poor prognosis, although the association was not significant. Our results were in agreement with the TCGA data, but there were some minor differences that might be attributed to the small sample size or incomplete case information in the data. Moreover, fluorescent in situ hybridization (FISH) or polymerase chain reaction (PCR) were used for XPO1 mRNA detection in the TCGA, whereas in our study immunohistochemistry was applied for protein detection. XPO1 might be a more powerful protein, but the clinical significance of XPO1 in DLBCL still needs to be demonstrated in larger numbers of studies. In the TCGA, we found XPO1 mutations in DLBCL, although they had no relationship with prognosis. Camusa [45] employed second-generation sequencing and digital PCR to evaluate DLBCL tissues and peripheral blood-related cytokines and found abnormal XPO1 expression. Similarly, Mareschal [46] used whole-exome sequencing for relapsed/refractory patients with DLBCL and observed XPO1 mutations. The XPO1 data in the TCGA and the network of related genes in DLBCL provide a basis for future research, although the mechanism of XPO1 in DLBCL needs to be ascertained experimentally.
Several studies have investigated the pathogenic mechanism of XPO1 in tumors. Kajiyama [47] demonstrated that XPO1 regulated the epithelial-mesenchymal transition (EMT) pathway in ovarian cancer, reducing cellular polarity and adhesion and thereby accelerating the infiltration and transfer of tumor cells. An XPO1 inhibitor suppressed the activation of the IGF-1R/AKT pathway by upregulating insulin-like growth factor-binding protein 5 (IGFBP5) in liposarcoma [48]. The tumor suppressor proteins FOXO, p53, and p27, which perform nucleo-cytoplasmic shuttling, play tumor-suppressive roles in the nucleus; a large proportion of tumor suppressor proteins were transported out of the nucleus when XPO1 was overexpressed, which promoted tumor cell proliferation [19,40]. XPO1 inhibitors down-regulated NF-κB expression and upregulated p53 expression in NHL cells [42]. Additionally, XPO1 inhibitors prevented the nuclear export of NF-κB-IκBα and prohibited NF-κB signaling in primary mediastinal B-cell lymphoma (PMBCL), hence inducing tumor cell apoptosis [46]. Zhou [30] found that XPO1 participated in the occurrence and progression of DLBCL in cooperation with NF-κBp50. Moreover, XPO1 regulated the PI3K-Akt pathway, which facilitated the proliferation of mantle cell lymphoma and prevented apoptosis [49]. However, because there has been limited research investigating XPO1 in DLBCL, determining its specific mechanism of action requires further exploration.
In conclusion, XPO1 protein expression was higher in DLBCL than in non-tumor lymphoid tissues. XPO1 overexpression was associated with an advanced clinical stage and a poor IPI score and was associated with a poor DLBCL prognosis. Additionally, the detection of XPO1 expression in DLBCL tissues could help achieve a better assessment of the patient’s condition and prognosis and provide evidence for the clinical diagnosis and treatment of DLBCL.
Acknowledgements
We would like to thank the Fund of the Key Programs of University Scientific Research of Guangxi Education Agency (No. ZD2014033), the Key Programs of Guangxi Natural Science Fund (No. 2015GXNSFDA139028), and the Promoting Project of Basic Capacity for University Young and Middle aged Teachers in Guangxi (2017). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge the cBioPortal for Cancer Genomics site (http://www.cbioportal.org/) and the TCGA Research Network for generating TCGA datasets (http://cancergenome.nih. gov/).
Disclosure of conflict of interest
None.
References
- 1.Krause G, Hassenruck F, Hallek M. Copanlisib for treatment of B-cell malignancies: the development of a PI3K inhibitor with considerable differences to idelalisib. Drug Des Devel Ther. 2018;12:2577–2590. doi: 10.2147/DDDT.S142406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu PP, Sun C, Cao X, Zhao X, Dai HJ, Lu S, Guo JJ, Fu SJ, Liu YX, Li SC, Chen M, McCord R, Venstrom J, Szafer-Glusman E, Punnoose E, Kiermaier A, Cheng G, Zhao WL. Immune characteristics of chinese diffuse large b-cell lymphoma patients: implications for cancer immunotherapies. EBioMedicine. 2018;33:94–104. doi: 10.1016/j.ebiom.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong QX, Lu TX, Liu C, Wang Z, Liang JH, Xu W, Li JY, Zhang ZH, Chen Q. Prevalence and clinicopathologic features of CD30-positive de novo diffuse large B-cell lymphoma in Chinese patients: a retrospective study of 232 cases. Int J Clin Exp Pathol. 2015;8:15825–15835. [PMC free article] [PubMed] [Google Scholar]
- 4.Board PDQPTE . Childhood non-hodgkin lymphoma treatment (PDQ(R)): health professional version. In: PDQ pediatric treatment editorial board, editor. PDQ cancer information summaries [Internet] Bethesda (MD): National Cancer Institute (US); 2002-2018. Aug 22, [Google Scholar]
- 5.Mu S, Ai L, Fan F, Qin Y, Sun C, Hu Y. Prognostic role of neutrophil-to-lymphocyte ratio in diffuse large B cell lymphoma patients: an updated dose-response meta-analysis. Cancer Cell Int. 2018;18:119. doi: 10.1186/s12935-018-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao HY, Wu B, Yan W, Gong ZM, Sun Q, Wang HH, Yang W. Microarray expression profiles of long non-coding RNAs in germinal center-like diffuse large B-cell lymphoma. Oncol Rep. 2017;38:1363–1372. doi: 10.3892/or.2017.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JM, Jang JY, Jeon YK, Paik JH. Clinicopathologic implication of microRNA-197 in diffuse large B cell lymphoma. J Transl Med. 2018;16:162. doi: 10.1186/s12967-018-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobashi A. Molecular pathogenesis of diffuse large B-cell lymphoma. J Clin Exp Hematop. 2016;56:71–78. doi: 10.3960/jslrt.56.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong W, Xu X, Zhu Z, Yang L, Du H, Xia Z, Yuan Z, Xiong H, Du Q, Wei Y, Li Q. Increased interleukin-17A levels promote rituximab resistance by suppressing p53 expression and predict an unfavorable prognosis in patients with diffuse large B cell lymphoma. Int J Oncol. 2018 doi: 10.3892/ijo.2018.4299. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo S, Hong JY, Yoon S, Yoo C, Park JH, Lee JB, Park CS, Huh J, Lee Y, Kim KW, Ryu JS, Kim SJ, Kim WS, Yoon DH, Suh C. Prognostic significance of serum beta-2 microglobulin in patients with diffuse large B-cell lymphoma in the rituximab era. Oncotarget. 2016;7:76934–76943. doi: 10.18632/oncotarget.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun F, Zhu J, Lu S, Zhen Z, Wang J, Huang J, Ding Z, Zeng M, Sun X. An inflammation-based cumulative prognostic score system in patients with diffuse large B cell lymphoma in rituximab era. BMC Cancer. 2018;18:5. doi: 10.1186/s12885-017-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Ding N, Wang X, Mi L, Ping L, Jin X, Liu Y, Ying Z, Xie Y, Liu W, Song Y, Zhu J. EP300 single nucleotide polymorphism rs20551 correlates with prolonged overall survival in diffuse large B cell lymphoma patients treated with R-CHOP. Cancer Cell Int. 2017;17:70. doi: 10.1186/s12935-017-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S, Sheng L, Xu K, Wang Y, Zhu H, Zhang P, Mu Q, Ouyang G. Anticancer effect of quinacrine on diffuse large Bcell lymphoma via inhibition of MSI2NUMB signaling pathway. Mol Med Rep. 2018;17:522–530. doi: 10.3892/mmr.2017.7892. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes J, Landsburg DJ. Small-molecule inhibitors for the treatment of diffuse large B cell lymphoma. Curr Hematol Malig Rep. 2018;13:356–368. doi: 10.1007/s11899-018-0467-5. [DOI] [PubMed] [Google Scholar]
- 16.Karmali R, Gordon LI. Molecular subtyping in diffuse large B cell lymphoma: closer to an approach of precision therapy. Curr Treat Options Oncol. 2017;18:11. doi: 10.1007/s11864-017-0449-1. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi UH, Senapedis W, Baloglu E, Unger TJ, Chari A, Vogl D, Cornell RF. Clinical implications of targeting XPO1-mediated nuclear export in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018;18:335–345. doi: 10.1016/j.clml.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Fu SC, Fung HYJ, Cagatay T, Baumhardt J, Chook YM. Correlation of CRM1-NES affinity with nuclear export activity. Mol Biol Cell. 2018;29:2037–2044. doi: 10.1091/mbc.E18-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizawa J, Kojima K, Hail N Jr, Tabe Y, Andreeff M. Expression, function, and targeting of the nuclear exporter chromosome region maintenance 1 (CRM1) protein. Pharmacol Ther. 2015;153:25–35. doi: 10.1016/j.pharmthera.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva G, Marins M, Chaichanasak N, Yoon Y, Fachin AL, Pinhanelli VC, Regasini LO, Dos Santos MB, Ayusso GM, Marques BC, Wu WW, Phue JN, Shen RF, Baek SJ. Trans-chalcone increases p53 activity via DNAJB1/HSP40 induction and CRM1 inhibition. PLoS One. 2018;13:e0202263. doi: 10.1371/journal.pone.0202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao WY, Yang YL, Yan H, Huang Q, Liu KJ, Zhang S. Phenethyl isothiocyanate suppresses the metastasis of ovarian cancer associated with the inhibition of CRM1-mediated nuclear export and mTOR-STAT3 pathway. Cancer Biol Ther. 2017;18:26–35. doi: 10.1080/15384047.2016.1264540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranganathan P, Kashyap T, Yu X, Meng X, Lai TH, McNeil B, Bhatnagar B, Shacham S, Kauffman M, Dorrance AM, Blum W, Sampath D, Landesman Y, Garzon R. XPO1 inhibition using selinexor synergizes with chemotherapy in acute myeloid leukemia by targeting dna repair and restoring topoisomerase iialpha to the nucleus. Clin Cancer Res. 2016;22:6142–6152. doi: 10.1158/1078-0432.CCR-15-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Ly C, Ishizawa J, Mu H, Ruvolo V, Shacham S, Daver N, Andreeff M. Combinatorial targeting of XPO1 and FLT3 exerts synergistic anti-leukemia effects through induction of differentiation and apoptosis in FLT3-mutated acute myeloid leukemias: from concept to clinical trial. Haematologica. 2018;103:1642–1653. doi: 10.3324/haematol.2017.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabi F, Adam P, Vincent K, Demontigny F, Parent S, Joncas FH, Asselin E. Inhibition of CRM1 activity sensitizes endometrial and ovarian cell lines to TRAIL-induced cell death. Cell Commun Signal. 2018;16:39. doi: 10.1186/s12964-018-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camus V, Miloudi H, Taly A, Sola B, Jardin F. XPO1 in B cell hematological malignancies: from recurrent somatic mutations to targeted therapy. J Hematol Oncol. 2017;10:47. doi: 10.1186/s13045-017-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner JG, Dawson JL, Grant S, Shain KH, Dalton WS, Dai Y, Meads M, Baz R, Kauffman M, Shacham S, Sullivan DM. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J Hematol Oncol. 2016;9:73. doi: 10.1186/s13045-016-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiecinska A, Ichimura K, Berglund M, Dinets A, Sulaiman L, Collins VP, Larsson C, Porwit A, Lagercrantz SB. Amplification of 2p as a genomic marker for transformation in lymphoma. Genes Chromosomes Cancer. 2014;53:750–768. doi: 10.1002/gcc.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholtysik R, Kreuz M, Hummel M, Rosolowski M, Szczepanowski M, Klapper W, Loeffler M, Trumper L, Siebert R, Kuppers R. Characterization of genomic imbalances in diffuse large B-cell lymphoma by detailed SNP-chip analysis. Int J Cancer. 2015;136:1033–1042. doi: 10.1002/ijc.29072. [DOI] [PubMed] [Google Scholar]
- 29.Trifonov V, Pasqualucci L, Dalla Favera R, Rabadan R. MutComFocal: an integrative approach to identifying recurrent and focal genomic alterations in tumor samples. BMC Syst Biol. 2013;7:25. doi: 10.1186/1752-0509-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou YQ, Chai L. NF-κBp50, JAB1 and CRM1 expression in diffuse large B cell lymphoma and their clinical significance. J of Wannan Medical College (In Chinese) 2012;31:92–95. [Google Scholar]
- 31.Ye ZH, Gao L, Wen DY, He Y, Pang YY, Chen G. Diagnostic and prognostic roles of IRAK1 in hepatocellular carcinoma tissues: an analysis of immunohistochemistry and RNA-sequencing data from the cancer genome atlas. Onco Targets Ther. 2017;10:1711–1723. doi: 10.2147/OTT.S132120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mo CH, Gao L, Zhu XF, Wei KL, Zeng JJ, Chen G, Feng ZB. The clinicopathological significance of UBE2C in breast cancer: a study based on immunohistochemistry, microarray and RNA-sequencing data. Cancer Cell Int. 2017;17:83. doi: 10.1186/s12935-017-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Chen E, You D, Song Y, Sun Z, Yue L. Both high expression of nucleophosmin/B23 and CRM1 predicts poorer prognosis in human gastric cancer. Apmis. 2016;124:1046–1053. doi: 10.1111/apm.12604. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Deng Y, He RQ, Li XJ, Ma J, Chen G, Hu XH. Upregulation of HOXA11 during the progression of lung adenocarcinoma detected via multiple approaches. Int J Mol Med. 2018;42:2650–2664. doi: 10.3892/ijmm.2018.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen WJ, Tang RX, He RQ, Li DY, Liang L, Zeng JH, Hu XH, Ma J, Li SK, Chen G. Clinical roles of the aberrantly expressed lncRNAs in lung squamous cell carcinoma: a study based on RNA-sequencing and microarray data mining. Oncotarget. 2017;8:61282–61304. doi: 10.18632/oncotarget.18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dekker JD, Park D, Shaffer AL 3rd, Kohlhammer H, Deng W, Lee BK, Ippolito GC, Georgiou G, Iyer VR, Staudt LM, Tucker HO. Subtype-specific addiction of the activated B-cell subset of diffuse large B-cell lymphoma to FOXP1. Proc Natl Acad Sci U S A. 2016;113:E577–586. doi: 10.1073/pnas.1524677113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu TX, Gong QX, Wang L, Fan L, Zhang XY, Chen YY, Wang Z, Xu W, Zhang ZH, Li JY. Immunohistochemical algorithm alone is not enough for predicting the outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Int J Clin Exp Pathol. 2015;8:275–286. [PMC free article] [PubMed] [Google Scholar]
- 38.Li PP, Feng LL, Chen N, Lu K, Meng XH, Ge XL, Lv X, Wang X. Metadherin interference inhibits proliferation and enhances chemo-sensitivity to doxorubicin in diffuse large B cell lymphoma. Int J Clin Exp Med. 2014;7:2081–2086. [PMC free article] [PubMed] [Google Scholar]
- 39.Turturro F. Constitutive NF-kappa B activation underlines major mechanism of drug resistance in relapsed refractory diffuse large b cell lymphoma. Biomed Res Int. 2015;2015:484537. doi: 10.1155/2015/484537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, Weiss RH. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. J Urol. 2013;189:2317–2326. doi: 10.1016/j.juro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendonca J, Sharma A, Kim HS, Hammers H, Meeker A, De Marzo A, Carducci M, Kauffman M, Shacham S, Kachhap S. Selective inhibitors of nuclear export (SINE) as novel therapeutics for prostate cancer. Oncotarget. 2014;5:6102–6112. doi: 10.18632/oncotarget.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X, Wang J, Shen Y, Zhang N, Wang S, Yao J, Shi Y. CRM1 as a new therapeutic target for non-Hodgkin lymphoma. Leuk Res. 2015;39:38–46. doi: 10.1016/j.leukres.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Tabe Y, Kojima K, Yamamoto S, Sekihara K, Matsushita H, Davis RE, Wang Z, Ma W, Ishizawa J, Kazuno S, Kauffman M, Shacham S, Fujimura T, Ueno T, Miida T, Andreeff M. Ribosomal biogenesis and translational flux inhibition by the selective inhibitor of nuclear export (SINE) XPO1 antagonist KPT-185. PLoS One. 2015;10:e0137210. doi: 10.1371/journal.pone.0137210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner JG, Kashyap T, Dawson JL, Gomez J, Bauer AA, Grant S, Dai Y, Shain KH, Meads M, Landesman Y, Sullivan DM. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IkappaBalpha and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget. 2016;7:78896–78909. doi: 10.18632/oncotarget.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camus V, Sarafan-Vasseur N, Bohers E, Dubois S, Mareschal S, Bertrand P, Viailly PJ, Ruminy P, Maingonnat C, Lemasle E, Stamatoullas A, Picquenot JM, Cornic M, Beaussire L, Bastard C, Frebourg T, Tilly H, Jardin F. Digital PCR for quantification of recurrent and potentially actionable somatic mutations in circulating free DNA from patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2016;57:2171–2179. doi: 10.3109/10428194.2016.1139703. [DOI] [PubMed] [Google Scholar]
- 46.Mareschal S, Dubois S, Viailly PJ, Bertrand P, Bohers E, Maingonnat C, Jais JP, Tesson B, Ruminy P, Peyrouze P, Copie-Bergman C, Fest T, Jo Molina T, Haioun C, Salles G, Tilly H, Lecroq T, Leroy K, Jardin F. Whole exome sequencing of relapsed/refractory patients expands the repertoire of somatic mutations in diffuse large B-cell lymphoma. Genes Chromosomes Cancer. 2016;55:251–267. doi: 10.1002/gcc.22328. [DOI] [PubMed] [Google Scholar]
- 47.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–283. [PubMed] [Google Scholar]
- 48.Garg M, Kanojia D, Mayakonda A, Said JW, Doan NB, Chien W, Ganesan TS, Chuang LS, Venkatachalam N, Baloglu E, Shacham S, Kauffman M, Koeffler HP. Molecular mechanism and therapeutic implications of selinexor (KPT-330) in liposarcoma. Oncotarget. 2017;8:7521–7532. doi: 10.18632/oncotarget.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang KJ, Wang M. Potential effects of CRM1 inhibition in mantle cell lymphoma. Chin J Cancer Res. 2012;24:374–387. doi: 10.3978/j.issn.1000-9604.2012.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]