Short abstract
We hypothesized that a mixture of blackberry fruit and leaf extracts may alleviate non-alcoholic fatty liver disease (NAFLD). Rats with diet-induced NAFLD were used to test the hypothesis and explore possible mechanisms. Male Sprague–Dawley rats were orally administered diets 51% of energy from fat and 450 mg dextrin/kg bw (NAFLD-control), 50% ethanol blackberry leaf extract (450 mg/kg bw; BL), 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), and milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Normal-control rats were fed low-fat diets with 450 mg dextrin/kg bw (20 En% fat diet) Body weight, visceral fat mass, liver triglycerides, serum cholesterol, triglyceride, non-esterified fatty acid, and insulin resistance were all elevated in rats in the NAFLD-control group compared to the normal-controls. Rats in the NAFLD-control group exhibited liver damage accompanied by increased oxidative stress and inflammation compared to the rats in the normal-control group. BL and BLF protected the NAFLD rats against the triglyceride and lipid peroxide accumulation, improved insulin sensitivity and dyslipidemia, and increased the antioxidant enzymes, SOD, and GSH-Px, to levels similar to the normal-control group. Further, BL and BLF ameliorated inflammation and hepatocyte damage compared to the NAFLD-controls, and they suppressed mRNA expressions of genes involved in triglyceride synthesis (FAS and SREBP-1c). BLF also modulated the gut microbiota by elevating Lactobacillus and Akkermansia in the feces from the cecum compared to the NAFLD-control group. The integrity of intestinal tissues was improved, and the number of goblet cells was elevated by BLF. In conclusion, BL and BLF prevented high-fat diet-induced liver damage by protecting against oxidation and inflammation-induced injury. BLF (human equivalent 1.3 g/day) might, therefore, be used as a therapeutic agent for NAFLD.
Impact statement
NAFLD is a diet-related metabolic disease with no good drug treatments. Therefore, dietary interventions are needed to alleviate NAFLD. This paper demonstrated that feeding a blackberry leaf and fruit mixture extract can alleviate diet-induced NAFLD in rats. Specifically, the blackberry extract, rich in flavonoids and anthocyanins decreased hepatic triglycerides and lipid peroxides, increased genes related to beta oxidation, decreased those involved fatty acid biosynthesis, alleviated oxidative stress, and suppressed pro-inflammatory cytokine release. The blackberry extract also alleviated gut dysbiosis that was associated with NAFLD by increasing the amount of Lactobacillus and Akkermansia in the feces. This research demonstrated that the extract of a common and inexpensive fruit can help alleviate NAFLD and associated intestinal dysbiosis at a dose equivalent to 1.3 g/day in humans. If this work can be duplicated in humans, it would provide a safe and inexpensive intervention to help alleviate NAFLD.
Keywords: Blackberry, non-alcoholic steatosis, intestinal integrity, Lactobacillus, Akkermansia
Introduction
The westernization of diets and lifestyles has increased in obesity and metabolic syndromes including non-alcoholic hepatic fatty liver disease (NAFLD) and steatohepatitis that is unrelated to alcohol consumption.1 Both of them involve excessive fat accumulation (>5% of liver weight) in the liver, and NAFLD is accompanied by hepatic inflammation which can progress to non-alcoholic steatohepatitis.1 Further, NAFLD occurs in about 60–80% of people suffering from obesity and in 10–20% of people without obesity.2 It is considerably more prevalent in middle-aged men than in women. Further, although the etiology of NAFLD and non-alcoholic steatohepatitis is not clearly understood, it is associated with an increased oxidative burden associated with increased hepatic fat metabolism.3 One strategy for preventing the occurrence of NAFLD is by modulating fat intake and decreasing oxidative stress.3,4
NAFLD is often associated with diseases related to excessive energy and fat consumption such as obesity and insulin resistance5 which cause the release of non-esterified fatty acids into circulation.6 High concentrations of non-esterified fatty acids lead to liver injury, and their utilization increases the production of hydrogen peroxide in the mitochondria in the liver.3 Oxidative stress caused by hydrogen peroxide results in increased production of lipid peroxides and the proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), and monocyte chemoattractant protein-13; and this increase stimulates the expression and activity of fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD-1), and acetyl-Co carboxylase (ACC).7–9 The increased oxidative stress and inflammation result in an exacerbation of NAFLD.7 Inflammation may subsequently cause NAFLD to progress to non-alcoholic steatohepatitis, liver cancer, and liver cirrhosis.
Recent studies have shown that obesity results in variations in the Firmicutes/Bacteroidetes ratio in the gut as an excessive energy intake including a low intake of dietary fiber can alter the nutrient status in the gut, which modulates the diversity and composition of gut bacteria.10 Most nutrients are absorbed in the small intestines and the non-digestible fiber goes to the large intestines where most bacteria live. The bacteria in the large intestine synthesize short-chain fatty acids when metabolizing the undigested fiber. The increase in short-chain fatty acids increases the secretion of peptide YY and glucagon-like-peptide-1, choline hydrolysis to dimethyl-nitrosamine precursors, suppresses bile acid production, and leads to increased inflammation.11 Excess sugar and lipids have been linked to intestinal and systemic inflammation which may in turn cause live inflammation and subsequent hepatic steatosis.11,12 Maintaining a healthy microbiome may help prevent the occurrence of NAFLD, which would decrease inflammation and oxidative stress.
Although preventive and therapeutic agents are needed for fatty liver diseases, only a few are currently available. Non-alcoholic fatty liver (>5% hepatic steatosis without liver injury) is quite common and usually managed by diet and lifestyle aimed at decreasing body weight and improving insulin sensitivity.13 If the fatty liver progresses to the subsequent stages of hepatitis, fibrosis, and cirrhosis, more aggressive treatment is needed, but there are no current FDA-approved drugs.14 However, vitamin E and perhaps pioglitazone may suppress inflammation in subjects with steatohepatitis as shown in the trial by Sanyal et al.14 Ursodeoxycholic acid, biphenyl dimethyl dicarboxylate, glutathione, silymarin, and a blend of flavonolignans from the milk thistle (Silybum marianum Gaertneri) and silymarin have also been used for regenerating a damaged liver.15–17 However, although the role of milk thistle in the treatment of NAFLD has been the subject of substantial research,16 its efficacy remains uncertain and there are no approved pharmaceutical or nutraceutical interventions for NAFLD despite numerous potential therapies have been studied.18 Therefore, better ingredients for treating fatty liver need to be studied.
Blackberry (Rubus fruticosus) leaves and fruits are reported to decrease insulin resistance, oxidative stress, and inflammation. Anthocyanins including cyanidin-3-glucoside and total alkaloids from blackberry fruits have protective effects against NAFLD in HepG2 cells and rats.19,20 In addition, suppressed nuclear factor kappa light chain enhancer of activated B cells signaling by blackberry results in an anti-inflammatory effect in rats with NAFLD.19–21 Blackberry leaves also contain flavonoids and their water extracts may have better anti-NAFLD properties. However, the extracts of blackberry leaves and the combination of extracts of blackberry fruits and leaves have not been used to examine the alleviation or prevention of NAFLD in most studies. Here, we hypothesized that the mixture of blackberry fruit and leaf extracts may alleviate NAFLD in an animal model. The hypothesis was examined in a diet-induced rat model of NAFLD using a diet high in fat and cholesterol, since this model may mimic human NAFLD linked to increased visceral fat.22
Materials and methods
50% ethanol extract of blackberry leaves and fruits
Freeze-dried blackberry fruits and leaves (Korea Prime Inc., Sungnam, Korea) were ground to a fine powder and extracted in for 24 h in five volumes of 50% ethanol at 25°C. Dried extract powders were then prepared by solvent removal by evaporation at 40°C and 20 hPa using a rotary evaporator at 30 r/min (Bunchi, Essen, Germany) followed by lyophilization in a freeze dryer (Il Sin Bio Base, Dongduchun, Korea). The yields for the blackberry fruits and leaves were 39.0 and 30.8%, respectively. The 50% ethanol extracts of BF and BL contained 12 ± 0.4 mg cyanidin-3-glucoside and 28.3 ± 1.2 mg ellagic acid, respectively, as shown in our previous study.23
Animals
Sprague–Dawley rats (aged 11 weeks and weighing 195 ± 11 g) were housed in separate stainless-steel cages in an environmentally controlled animal facility (23°C, 12-h light/dark cycle). The research protocol was consistent with international guidelines and approved by the Animal Care and Use Review Committee of Hoseo University, Korea (No. HSIACUC-18–129).
Diet preparation and experimental design
The modified AIN-93 high-fat diet utilized carbohydrate (20% sucrose and 13% starch) for 33% of energy, protein (casein) for 16% of energy, and the remaining 51% of energy was from fats (49% lard and 2% corn oil). Cellulose (1.5%) was added for dietary fiber. The 50% ethanol blackberry leaf and fruit extracts and their mixture (2:1) were added to the high-fat diet. The high dosage (90 µg/mL) of individual blackberry leaf and fruit extracts reduces triglyceride deposition and the mixture (30 µg/mL) of blackberry leaf and fruits (2:1) had a similar effect on triglyceride accumulation to that of 90 µg/mL of individual blackberry leaves or fruits in HepG2 cells treated with palmitate. The treatment amount of the blackberry extracts was extrapolated from a cell-based study.23 A 1.5% extract of either blackberry leaf and fruit was supplemented into the high-fat diet for testing the extracts individually. A 0.5% mixture—one-third of the individual blackberry leaf and fruit extract—was supplemented in the high-fat diet for testing the combined effects. The amount of the mixture added into a high-fat diet was the same as the milk thistle, which was used as a positive-control. Since the blackberry extracts contained polyphenols and soluble fibers, dextrin was added in the control diet instead of the blackberry extracts. The control and experimental diets were designed to provide equivalent nutrient contents.
Sixty rats were randomly assigned to six groups: (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg body weight; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-control), and (5) milk thistle extracts (150 mg/kg bw; positive-control). (6) The remaining 10 rats were fed a low- (20 En%) fat diet as a normal-control group. Throughout the entire 12-week experimental period, the rats had free access to water and their respective diets.
Overnight-fasted serum glucose concentrations, feed and water intakes, and body weights were recorded every Tuesday at 10:00 a.m. Insulin sensitivity was evaluated using the homeostasis model assessment estimate of insulin resistance (HOMA-IR). At the conclusion of the study, rats were anesthetized with ketamine and xylazine (100 and 10 mg/kg body weight, respectively). Epididymal and retroperitoneal fat pads and uteri were surgically removed and weighed. Hepatic insulin signaling was assessed by injecting human insulin (5 U/kg body weight) into the inferior vena cava. Serum samples were then stored at –70°C for subsequent biochemical analyses. Liver damage markers in serum, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were determined using colorimetric kits (Asan Pharmaceutical Company Seoul, Korea). Livers were removed and stored at –70°C for further study. An ELISA kit (Mybiosource.com, San Diego, CA, USA) was used to determine the serum non-esterified fatty acids (NEFA) concentrations.
Liver glycogen was determined in livers homogenized in 1.5 N perchloric acid treated with α-amyloglucosidase to hydrolyze the glycogen. The glucose concentrations were measured in supernatants using a glucose oxidase kit (Asan Pharmaceutical, Seoul, Korea)24 and liver glycogen contents were calculated from the glucose concentrations. Hepatic triglycerides were extracted using chloroform-methanol (2:1, vol/vol) as the solvent, and they were resuspended in pure chloroform.25 Triacylglycerol concentrations were determined using a Trinder kit purchased from Asan Pharmaceutical Co. (Seoul, Korea) after evaporating chloroform and suspending the residues in PBS with 0.1% Triton X-100.
Antioxidant status in the liver
Hepatic lipid peroxidation was estimated using a thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical, Ann Arbor, MI, USA). Hepatic TNF-α levels were assayed by ELISA (R & D Systems, Minneapolis, MN and Amersham Biosciences, Piscataway, NJ, USA, respectively). Antioxidant enzyme activities, Cu/Zn superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were determined in liver lysates by using colorimetry kits (Cayman Chemical, Ann Arbor, Michigan, USA and Biovision, Milpitas, CA, USA), respectively.26 One unit of each enzyme activity was defined as the amount required to achieve 50% inhibition and the enzyme activity was normalized to mg protein in the lysate. Hepatic GSH concentrations were determined by using a GSH assay kit (Sigma Aldrich, St. Louis, MO, USA).
Isolation of liver total RNA and real-time PCR
Liver tissues ground to a fine powder with a cold steel mortar and pestle were mixed with a monophasic phenol and guanidine isothiocyanate solution (Trizol reagent; Gibco-BRL, Rockville, MD, USA) to extract the total RNA which was used to synthesize cDNA by PCR. cDNA in equal amounts was added to SYBR Green mix (Bio-Rad, Richmond, CA, USA) and amplified using RT-PCR (Bio-Rad). Gene expressions were normalized to β-actin. Differences in gene expressions related to hepatic fatty acid synthesis and oxidation were assessed by using RT-PCR primers for sterol regulatory element-binding protein (SREBP)-1c, FAS, carnitine palmitoyl transferase (CPT)-1, TNF-α, interleukin (IL)-1β, and β-actin26,27 with at least four replicates per group.
Histological analysis
Liver and intestine samples were fixed in 10%-buffered neutral formaldehyde and embedded in paraffin wax. Hematoxylin and eosin-(H–E) stained histological sections (6 μm thick) were used to quantify liver damage and to examine the changes in intestinal tissue. Glycogen and mucin levels were respectively measured using periodic acid–Schiff (PAS) and Alcian blue staining in the liver and intestinal tissues. The liver damage and glycogen contents assessments at 400× magnification used two sections randomly selected from six consecutive sections. Liver damage scores were determined by summing each item (nucleus size and shape, cell size and arrangement, and macrophage number) in a histological scoring system.28 Each item received scores of 0 (no change), 1 (mid), 2 (moderate), or severe (3), with higher scores indicating more damage. Furthermore, glycogen contents were quantified according to red color intensity in PAS-stained liver tissues; lower scores indicated greater glycogen contents. In intestinal tissues, H–E-stained sections were used to measure the area and integrity of intestinal tissues. Goblet cell numbers—an index of mucin production—were measured in the Alcian blue-stained section.
Next-generation sequencing of gut microbiome
Gut microbial composition was quantified in feces by analyzing metagenome sequencing using next-generation sequencing. Bacterial DNA extractions from fecal samples in the cecum of each rat were obtained using a power water DNA isolation kit (MoBio, Carlsbad, CA, USA). Libraries were prepared using PCR products as described in the GS FLX plus library prep guide.25 Mixtures of DNA capture beads and library-beads were vigorously shaken in a TissueLyser II (Qiagen, Valencia, CA, USA) to create “microreactors” containing both the amplification mix and a single bead. The emulsion was dispensed into a 96-well plate for PCR amplification using 16S universal primers in the FastStart™ High Fidelity PCR System (Roche, Basel, Switzerland). Fecal bacteria DNA was sequenced using Genome Sequencer FLX plus (454 Life Sciences) by Macrogen Ltd. (Seoul, Korea), as reported previously.29
Mothur v.1.36 was used for 16S amplicon sequencing.25 We utilized Miseq standard operating procedure for identification and counting of the bacteria in all fecal samples. All sequences were assigned taxonomic classifications using Greengenes 13_8_99 and any sequence identified as mitochondrial, Eukaryota, or unknown was removed. We conducted the picking of operational taxonomic units (OTUs) delimited at 98% identity, which was taxonomically classified by consensus using Greengenes 13_8_99. A relaxed neighbor-joining tree with one representative sequence per OTU was obtained with Clearcut after calculating uncorrected pairwise distances between aligned reads.
Statistical analyses
SAS (version 7.0) was used for data analyses and the results are expressed as means ± standard deviations. Distributions of all parameters were checked by the SAS statement of proc univariate and normal distributions were confirmed for each parameter. Accordingly, one-way ANOVA was used to compare the groups when the results were measured only once at the conclusion of the study. Tukey’s test was used for multiple comparisons with significance accepted at P < 0.05.
Results
Body, fat, liver weights, and food consumption
The NAFLD-control group had significantly higher final body weights than that in the normal-control group. However, blackberry treatments did not alter the body weight compared to that in the NAFLD-control group (Table 1). NAFLD-controls did experience greater weight gain during the 12 weeks than did the normal-control group, but the BL and positive-control groups gained less than the NAFLD-control group. Epidydimal and retroperitoneal fat masses in the NAFLD-control group were significantly greater than that in the normal-control group (Table 1). The BL group exhibited the lowest increase in fat mass among all treatment groups; however, final fat mass was still greater than that in the normal-control group. BLF and positive-control had less epidydimal and retroperitoneal fat masses compared to those of the NAFLD-control group, but still had more than the normal-control group (Table 1). The energy consumption was much higher in the NAFLD-controls compared to the normal-control group; however, there were no significant difference among the blackberry-treated groups. Therefore, differences in energy expenditure rather than energy intake may account for the lower increase in the body weight in the BL and positive-control groups. Rats had about 450 mg of BL or BF and 150 mg of BLF per kg body weight, respectively, each day (Table 1). Their intakes of BL, BF, and BLF were 3.8, 3.8 and 1.3 g per day, respectively, as human equivalent dose.
Table 1.
Body weight and glucose metabolism.
| NAFLD-C | BL | BF | BLF | Positive-C | Normal-C | |
|---|---|---|---|---|---|---|
| Body weight at 12 week (g) | 521 ± 38a | 513 ± 41a | 534 ± 44a | 533 ± 42a | 518 ± 47a | 428 ± 43b |
| Weight gain during 12 weeks (g) | 288 ± 13a | 270 ± 14b | 290 ± 17a | 289 ± 19a | 273 ± 18ab | 196 ± 14c |
| Epididymal fat pads (g) | 15.2 ± 1.7a | 10.6 ± 0.6c | 13.6 ± 1.3b | 13.1 ± 1.9b | 12.5 ± 1.6b | 7.3 ± 0.6c |
| Retroperitoneal fat (g) | 19.8 ± 1.8a | 15.7 ± 0.74c | 17.1 ± 1.3b | 15.7 ± 1.7c | 16.0 ± 1.4bc | 9.4 ± 1.2d |
| Caloric intake (kcal/day) | 86.9 ± 9.3a | 84.5 ± 3.9a | 89.6 ± 3.9a | 90.1 ± 4.7a | 87.9 ± 5.5a | 45.0 ± 4.6b |
| BL intake (mg/kg/ bw/day) | 0 | 458 ± 12 | 0 | 107 ± 12 | 157 ± 18 | 0 |
| BF intake (mg/kg/ bw/day) | 0 | 0 | 469 ± 13 | 53.3 ± 2.8 | 0 | 0 |
Note: Values are means ± standard deviation (n = 10). Rats consumed high-fat diets containing (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-control) and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Additionally, rats had low-fat diet (20 En% fat diet) with 450 mg dextrin/kg bw as a normal-control.
a,b,c,d Means on the same row with different letters were significantly different by Tukey’s test at P < 0.05.
Liver morphometry and histology
The NAFLD-control rats had enlarged and steatotic livers, unlike the normal-control group; BL, BF, and BLF protected against liver enlargement and fat accumulation. Compared to the normal-control group, liver weights were elevated in the NAFLD-control group, but BL and BLF partially protected against the liver enlargement (Table 2).
Table 2.
Hepatic lipid deposition, lipid peroxides, activities of antioxidant enzymes and proinflammatory cytokines.
| NAFLD-C | BL | BF | BLF | Positive-C | Normal-C | |
|---|---|---|---|---|---|---|
| Hepatic TG (µg/mg protein) | 358 ± 16a | 301 ± 13c | 300 ± 13c | 308 ± 14c | 321 ± 15b | 241 ± 14d |
| Hepatic cholesterol (µg/mg protein) | 180 ± 12a | 150 ± 11c | 172 ± 10a | 159 ± 9b | 158 ± 10b | 147 ± 10c |
| Hepatic glycogen (µg/mg protein) | 37.1 ± 1.3d | 44.2 ± 2.4b | 41.0 ± 2.7c | 45.8 ± 2.6b | 43.9 ± 3.0bc | 52.1 ± 4.7a |
| Hepatic MDA (nmol/mg protein) | 26.8 ± 1.6a | 23.3 ± 2.2b | 24.1 ± 1.9b | 20.7 ± 1.3c | 24.0 ± 2.0b | 24.3 ± 2.2b |
| Hepatic SOD (U/mg protein) | 31.7 ± 2.7c | 36.5 ± 3.1ab | 33.4 ± 3.1b | 37.5 ± 3.7a | 35.7 ± 2.9ab | 35.9 ± 3.1ab |
| Hepatic GSH peroxide (U/mg protein) | 54.1 ± 4.9b | 65.7 ± 5.3ab | 61.5 ± 5.5b | 68.6 ± 5.6a | 65.1 ± 5.3ab | 64.7 ± 5.8ab |
| Hepatic GSH (umol/g protein) | 20.5 ± 1.8c | 26.2 ± 1.6b | 25.7 ± 2.1b | 28.5 ± 2.3a | 27.2 ± 3.1ab | 28.9 ± 2.9a |
| Hepatic TNF-α (pg/g tissue) | 9.8 ± 0.9a | 8.0 ± 0.7bc | 8.8 ± 0.8b | 7.4 ± 0.8c | 7.8 ± 0.7c | 6.8 ± 0.7d |
Note: Values are means ± standard deviation (n = 10). Rats consumed high-fat diets containing (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-control) and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Additionally, rats had low-fat diet (20 En% fat diet) with 450 mg dextrin/kg bw as a normal-control.
a,b,c,d Means on the same row with different letters were significantly different by Tukey’s test at P < 0.05.
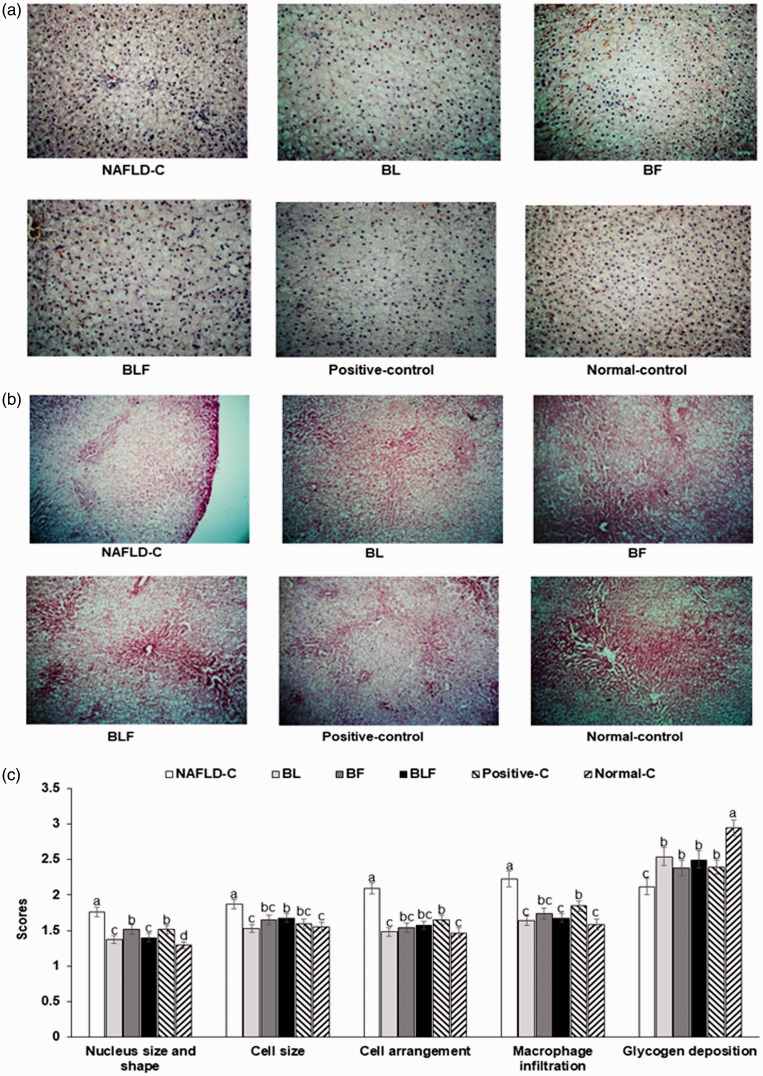
H–E staining revealed that the liver tissue morphology deteriorated more in NAFLD-control rats than normal-control rats (Figure 1(a) and (c)). The nuclei and cell sizes were larger than normal, and the cell arrangement and shape were irregular in the NAFLD-controls Figure 1(a) and (c)). These changes in the NAFLD-control group were associated with ballooning degeneration, an abnormal cell swelling that can result in liver parenchymal cell death due to enlarged cell size. BL and BLF partially protected against the enlargement of the nucleus and the cell size, and their protection was better than that of the positive control (Figure 1(c)).
Figure 1.
Liver morphological and histological characteristics. (a) Paraffin-embedded liver sections stained with hematoxylin and eosin (H–E) (magnification: ×200). (b) Paraffin-embedded liver sections stained with periodic acid–Schiff (PAS) (magnification: ×200). (c) Scores of the nucleus and cell structure, cell arrangement, macrophage infiltration, and glycogen deposition. Each bar or dot and error bar represents the mean±SD (n = 10).
Rats consumed high-fat diets supplemented with (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-C), and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Rats had low-fat diets (20 En% fat diet) containing 450 mg dextrin/kg bw as the normal-control group. a,b,c,d Bars with different letters were significantly different by Tukey’s test at P < 0.05. (A color version of this figure is available in the online journal.)
Cell damage is exacerbated by macrophage infiltration, which was elevated in the NAFLD-control group. All treatments inhibited macrophage infiltration, and BL and BLF had better inhibition of macrophage infiltration than the BF and positive control treatment (Figure 1(c)). Thus, BL and BLF protected against the high-fat-diet-induced liver damage. The glycogen deposition visualized by PAS staining was an indicator of liver damage (Figure 1(b) and (c)). Hepatic glycogen deposition was lower in the NAFLD-controls than in the normal-controls. Although the treatments increased the deposition, it was still less than that in the normal-control group. Consistent with the PAS staining results, the direct measurement of hepatic glycogen levels revealed low values in the NAFLD-control group that were increased with blackberry treatments (Table 2).
In contrast to glycogen accumulation, NAFLD-controls had elevated hepatic triglyceride and cholesterol levels (Table 2). All blackberry extract treatments lowered the hepatic triglyceride deposition to levels below than that of the positive-control group. Hepatic cholesterol accumulation was highest in the NAFLD-control and BL and BLF, but not BF, prevented accumulation similar to the positive-control group (Table 2).
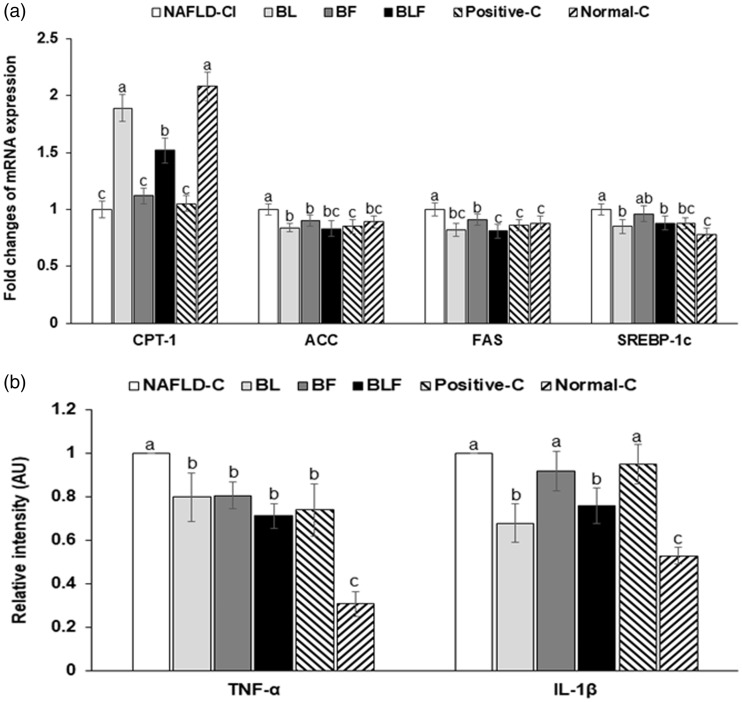
The deposition of triglyceride is defined as the sum of triglyceride synthesis and oxidation. CPT-1, the rate-limiting enzyme of fatty acid oxidation, facilitates the transport of fatty acids from the cytosol to mitochondria matrix across the mitochondria inner membrane. The CPT-1 expression was suppressed in the NAFLD-control (Figure 2(a)), but BL and BLF increased its expression; BL increased it to similar levels as the normal-control group. ACC, FAS, and SREBP-1c expressions were elevated in the NAFLD-control, but BL and BLF protected against the higher expressions of ACC and FAS mRNA, with similar levels as the positive-control group (Figure 2(a)). BL and BLF also decreased SREBP-1c, but not to the same extent as in normal-controls (Figure 2(a)).
Figure 2.
Relative gene expression related to fatty acid utilization and synthesis and inflammation. (a) Fold changes of mRNA expression of genes related to fatty acid synthesis. (b) Fold changes of mRNA expression of genes related to inflammation. Each bar or dot and error bar represents the mean±SD (n = 4). Rats consumed high-fat diets supplemented with (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-C), and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Rats had low-fat diets (20 En% fat diet) containing 450 mg dextrin/kg bw as the normal-control group. a,b,c,d Bars with different letters were significantly different by Tukey’s test at P < 0.05.
Liver damage and oxidative stress
High-fat diet-induced liver damage is accompanied by oxidative stress and inflammation. Oxidative stress is regulated by anti-oxidant enzymes and antioxidants. Hepatic malondialdehyde accumulation—an index of lipid peroxide level—was significantly higher in the NAFLD-control group than in the other groups, and it was significantly lower in the descending order of the NAFLD-control, BF, positive-control = BL, and BLF (Table 2). BF and BL lowered the hepatic malondialdehyde to similar levels as in the normal-controls, and BLF decreased it to less than that in the normal-controls. The hepatic SOD activity was lower in the NAFLD-control compared to that in normal-control group, and it was increased after the treatments with blackberry extracts (Table 2). BL and BLF increased it to as much as the positive and normal controls, Hepatic GSH-Px activity and GSH levels were low in the NAFLD-control group, but they were increased by the treatments with blackberry extracts, and BLF restored them to normal levels (Table 2).
Hepatic TNF-α and IL-1β mRNA expressions—indices of inflammation—were elevated in the NAFLD-control group, but blackberry extracts decreased hepatic TNF-α and IL-1β mRNA expressions as effectively as the positive control (Figure 2(b)). BLF decreased their expression the most and it was similar to that in the normal-control group (Figure 2(b)). Similar to the hepatic TNF-α expression, the TNF-α protein level in the liver was highly elevated in the NAFLD-control group, and it decreased in the descending order of NAFLD-control, BF, BL, positive-control, BLF, and normal-control groups. BLF reduced the level to as much as in the normal-control (Figure 2(b)). TNF-a protein levels in the liver showed similar patterns of mRNA expression levels (Table 2).
NAFLD-control rats exhibited significantly elevated serum AST and ALT levels (Table 3). Rats that were administered BL, BF, and BLF had significantly lower serum ALT and AST levels, in comparison to the NAFLD-control group, and their decrease was greater than that in the positive-control group (Table 3). Blackberry extracts decreased serum AST and ALT, demonstrating their protection against liver damage induced by a high-fat diet (Table 3).
Table 3.
Liver damage index and lipid profiles in the circulation.
| NAFLD-C | BL | BF | BLF | Positive-C | Normal-C | |
|---|---|---|---|---|---|---|
| Serum AST (IU/L) | 50.3±3.1a | 38.5±2.3c | 44.8±3.0b | 37.2±2.7c | 44.5±2.8b | 41.3±2.0bc |
| Serum ALT (IU/L) | 38.6±2.5a | 22.5±2.1c | 26.4±1.8b | 19.8±2.1c | 26.0±2.6bc | 20.4±2.0c |
| Serum total-C (mg/dL) | 105.5±9.0a | 78.0±3.2d | 85.3±4.7c | 92.6±5.8b | 90.5±6.1bc | 90.2±7.8bc |
| Serum HDL-C (mg/dL) | 13.2±0.7 | 13.3±0.8 | 12.8±1.2 | 13.2±0.9 | 13.8±1.1 | 13.8±0.9 |
| Serum LDL-C (mg/dL) | 62.9±3.4a | 50.6±2.6b | 51.0±3.8b | 46.6±3.9c | 51.2±3.1b | 61.8±3.1a |
| Serum TG (mg/dL) | 79.7±4.8a | 54.6±3.4c | 62.6±4.7b | 70.1±5.1a | 66.4±4.6ab | 64.1±3.2b |
| Serum glucose (mg/dL) | 122±10 | 119±8 | 122±9 | 119±6 | 121±6 | 114±8 |
| Serum insulin (ng/mL) | 0.62±0.08a | 0.47±0.07b | 0.56±0.09ab | 0.53±0.08b | 0.55±0.07ab | 0.52±0.08b |
| HOMA-IR | 8.4±0.9a | 6.2±0.8c | 7.6±1.0ab | 7.0±0.9bc | 7.4±0.8b | 6.9±0.9bc |
| Serum NEFA | 0.74±0.10a | 0.56±0.08bc | 0.61±0.09b | 0.58±0.08bc | 0.60±0.09b | 0.51±0.08c |
Note: Values are means±standard deviation (n = 10). Rats consumed high-fat diets containing (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-control) and (5) milk thistle extracts(150 mg/kg bw; positive-control) for 12 weeks. Additionally, rats had low-fat diet (20 En% fat diet) with 450 mg dextrin/kg bw as a normal-control.
a,b,c,d Means on the same row with different letters were significantly different by Tukey’s test at P < 0.05.
Lipid profiles and liver triglyceride accumulation
Serum total and LDL cholesterol concentrations were significantly elevated in the NAFLD-control group (Table 3). BL decreased both concentrations among all groups, and BF and BLF also lowered the levels to as much as that in the positive and normal-control groups. Serum HDL cholesterol did not differ significantly among the groups (Table 3). Serum triglyceride levels exhibited similar patterns as serum total and LDL-cholesterol; BL lowered the concentrations the most among all groups. Thus, BL alleviated dyslipidemia (Table 3).
There were no significant differences in fasting serum glucose concentrations among the groups (Table 3). Fasting serum insulin levels were highest in the NAFLD-control group, but were lower in rats given BL and BLF, and they were similar to those in the positive and normal-control rats (Table 3). HOMA-IR was much higher in the NAFLD-control group than other groups, and it decreased in the descending order of NAFLD-control, BF, positive-control, BL, BLF, and normal-control groups (Table 3). Fasting serum NEFA levels exhibited a similar pattern as that of HOMA-IR (Table 3).
Glucose metabolism
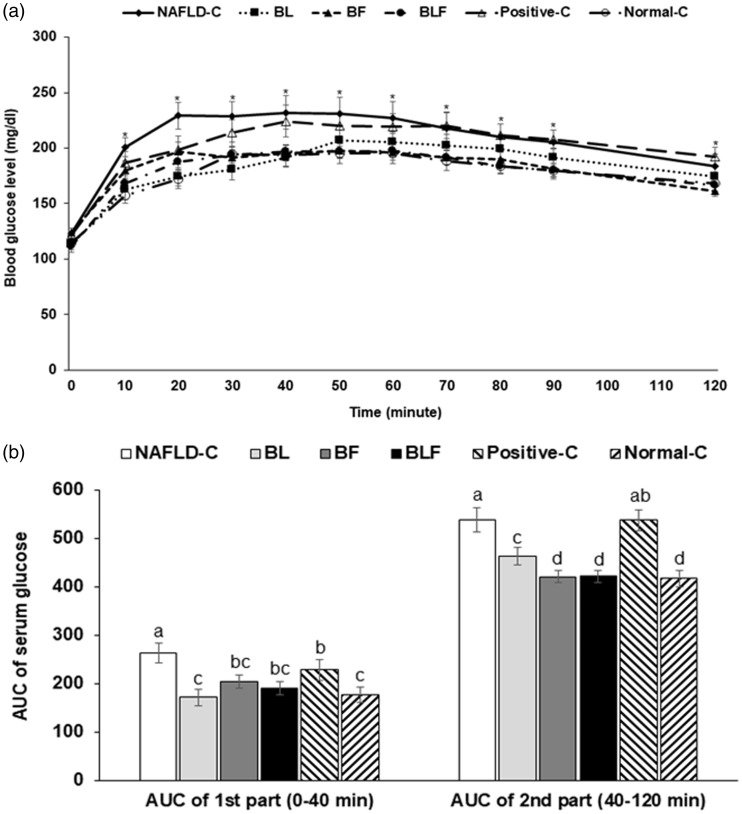
Serum glucose concentrations were consistently higher in the NAFLD-control group than in the other groups during the oral glucose tolerance test (OGTT), indicating that the rats in the NAFLD-control group were glucose intolerant. Glucose tolerance improved in BL, BF, and BLF groups; however, no improvement was evident for the positive-control group (Figure 3(a)). The area under the curve (AUC) of both phases of OGTT was highest in the NAFLD-control, and all treatments decreased the first and second phase AUCs (Figure 3(b)). BL and BLF lowered the first phase AUC, and the BF and BLF treatments decreased the second phase to as much as that of the normal-control group. However, second phase AUC was not lowered by the positive-control treatment (Figure 3(b)).
Figure 3.
Serum glucose levels during an oral glucose tolerance test. (a) Changes of serum glucose concentrations in 16-h fasted rats after an oral challenge of 2 g glucose/kg bw. (b) The area under the curve (AUC) of serum glucose calculated in the first (0–40 min) and second phases (40–120 min). Each bar or dot and error bar represents the mean±SD (n = 10). Rats consumed high-fat diets supplemented with (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-C), and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Rats had low-fat diets (20 En% fat diet) containing 450 mg dextrin/kg bw as the normal-control group. a,b,c,d Bars with different letters were significantly different by Tukey’s test at P < 0.05.
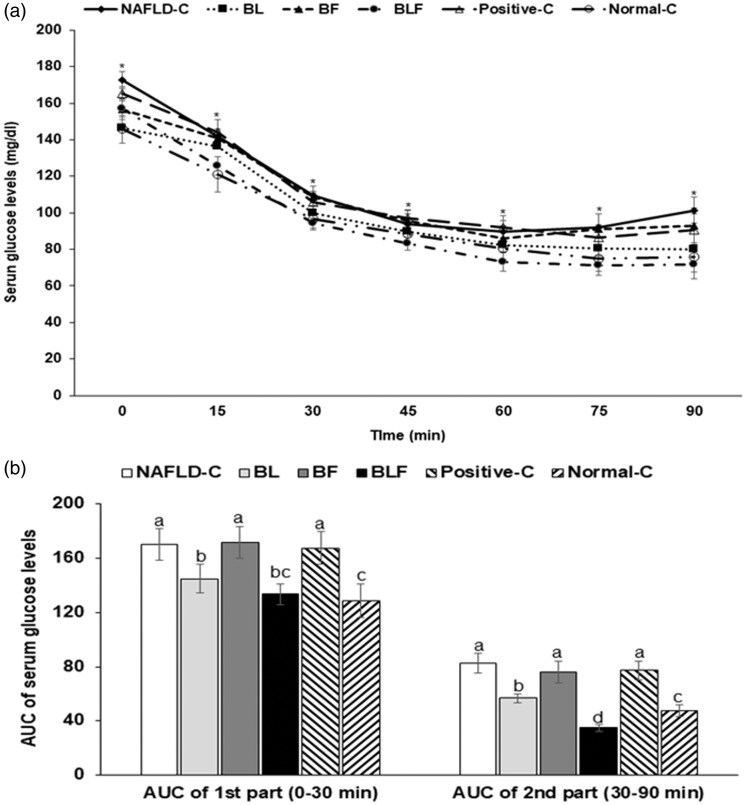
The changes in the serum glucose concentrations after intraperitoneal insulin tolerance test (IPITT), and following 6 h of food deprivation, are shown in Figure 4(a). Serum glucose concentrations were highest in the NAFLD-control group. All rats showed a marked decrease in the serum glucose concentrations until 30–45 min after insulin injection; after that glucose levels gradually declined or remained the same in most groups, except the NAFLD-control group (Figure 4(a)). However, serum glucose concentrations rebounded in the NAFLD-control rats at 45 min (Figure 4(a)). The first phase AUC (0–30 min) was highest in the NAFLD-control rats during IPITT; however, it was not significantly different among the treatments (Figure 4(b)). The second phase AUC was much higher in the NAFLD-control group than that in the normal-control and BL groups; further, although BLF reduced the AUC, it was higher than that of the normal-controls (Figure 4(b)).
Figure 4.
Changes of serum glucose levels during insulin tolerance test and hepatic insulin signaling. (a) Changes of serum glucose concentrations after 1 U insulin/kg bw into subcutaneous injection after 6 h food deprivation. (b) The area under the curve (AUC) of serum glucose levels calculated in the first (0–30 min) and second phases (30–90 min). Each bar or dot and error bar represents the mean±SD (n = 10). Rats consumed high-fat diets supplemented with (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-C), and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Rats had low-fat diet (20 En% fat diet) containing 450 mg dextrin/kg bw as the normal-control group. a,b,c,d Bars with different letters were significantly different by Tukey’s test at P < 0.05.
Intestinal tissues
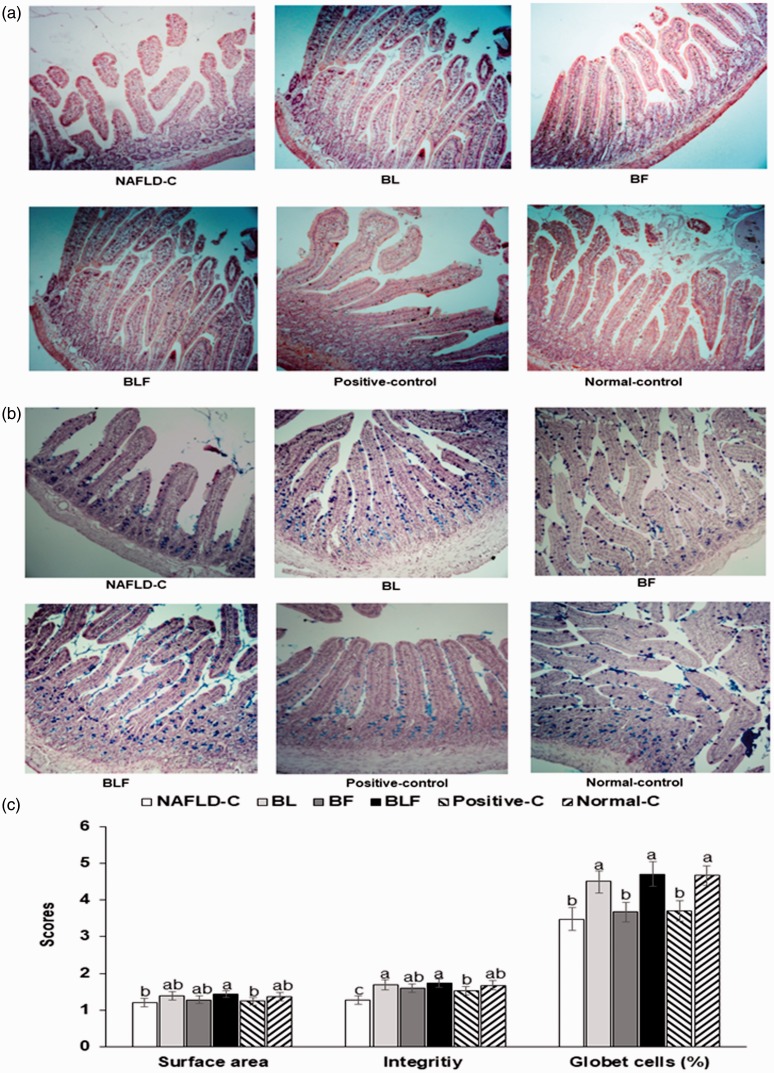
The NAFLD-control group had a lower intestinal surface area than the other groups, and they increased in the ascending order of control, BF = positive-control, BL, BLF, and normal-control groups (Figure 5(a) and (c)). The integrity of the intestinal tissues was deteriorated in the NAFLD-control group. The integrity was improved in the BL, BF, and BLF groups to as much as those of normal controls. The number of goblet cells secreting mucin was lower in the NAFLD-control group, and it was increased in the BL, BF, and BLF groups; the increase for the BLF group was the same as that for the normal-control group (Figure 5(b) and (c)).
Figure 5.
Histology of intestinal tissues. (a) Staining with H–E in the paraffin-embedded section of intestinal tissues (magnification: ×100). (b) Staining with PAS in the paraffin-embedded section of intestinal tissues (magnification: ×100). (c) Scores of the surface area, integrity and the percentage of goblet cells. Each bar or dot and error bar represents the mean±SD (n = 10). Rats consumed high-fat diets supplemented with (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-C), and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Rats had low-fat diet (20 En% fat diet) containing 450 mg dextrin/kg bw as the normal-control group. a,b,c,d Bars with different letters were significantly different by Tukey’s test at P < 0.05. (A color version of this figure is available in the online journal.)
Gut microbiome
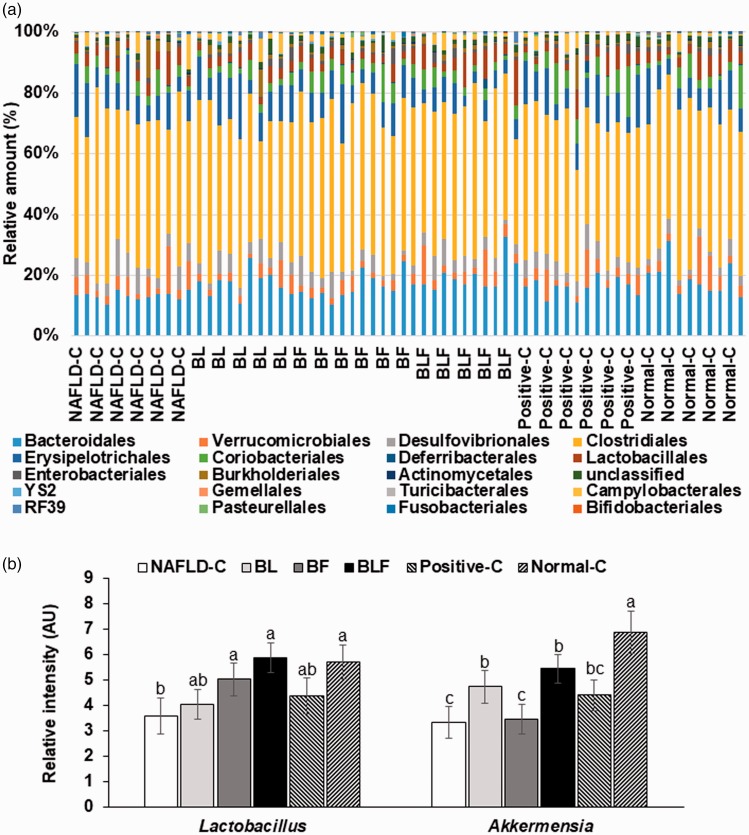
The order level bacterial distribution was not significantly different among the groups (Figure 6(a)). The major bacteria included Bacteroidales, Clostridiales, Erysipelotrichales, Desulfovibrionales, Lactobacillales, and Deferribacterales at the order level (Figure 6(a)). Akkermansia counts were higher in the BL, BF, and BLF groups then the NAFLD-control group (Figure 6(b)). Lactobacillus contents of BLF rats were much higher than those of other groups, with counts that were similar to the normal-controls (Figure 6(b)).
Figure 6.
Profiles of gut microbiome using the NGS analysis of gut microbial DNA. (a) The proportion of taxonomic assignments [order] for gut microbiomes. (b) The relative amounts of Lactobacillus and Akkermansia in the feces. Each bar or dot and error bar represents the mean±SD (n = 10). Rats consumed high-fat diets supplemented with (1) 50% ethanol blackberry leaf (450 mg/kg bw; BL), (2) 50% ethanol blackberry fruit extract (450 mg/kg bw; BF), (3) the mixture of blackberry leaf and fruit extracts (2:1; 150 mg/kg bw; BLF), (4) dextrin (450 mg/kg bw; NAFLD-C), and (5) milk thistle extracts (150 mg/kg bw; positive-control) for 12 weeks. Rats had low-fat diet (20 En% fat diet) containing 450 mg dextrin/kg bw as the normal-control group. a,b,c,d Bars with different letters were significantly different by Tukey’s test at P < 0.05. (A color version of this figure is available in the online journal.)
Discussion
Various factors including diets high in fat, fructose, and alcohol initiate hepatic triglyceride accumulation. When liver fat exceeds 5% of the organ weight, it is diagnosed as fatty liver.30 While the incidence of NAFLD is increasing in parallel with the global increase in obesity, the mechanism remains unclear. We hypothesized that a mixture of blackberry fruit and leaf extracts would help alleviate NAFLD, and tested the hypothesis in rats fed diets high in fat and cholesterol. We also examined the mechanism involved. The high fat and cholesterol diets induced NAFLD; a lower dosage of BLF than individual BL and BF partially protected against NAFLD by suppressing the mRNA expression of genes involved with fatty acid synthesis and potentiating antioxidant enzyme gene expression. Interestingly, BLF increased the amount of Lactobacillus and Akkermansia in the gut and enhanced the integrity of intestinal tissues and the percentage of goblet cells in the intestines. Therefore, BLF may be a therapeutic candidate for NAFLD.
Blackberry leaves and fruits contain flavonoids and anthocyanins that were extracted with 50% ethanol in the present study. Wang et al.20 showed that blackberry anthocyanin-rich extracts exhibit the largest decrease in fat accumulation in oleic acid-induced steatosis of HepG2 cells and the highest inhibition of reactive oxygen species generation among various berries; this may be attributed to the presence of different anthocyanins. Anti-oxidant activity may be one important mechanism by which the extracts protected against NAFLD since vitamin E has been demonstrated to be protective against NAFLD in humans.14 Cyanidin 3-O-glucoside has an inhibitory effect on triglyceride deposition that is more than that of delphinidin 3-glucoside, pelargonidin 3-glucoside, and malvidin 3-glucoside in HepG2 cells.20 In the present study, BF and BL contained cyanidin 3-glucoside and they suppressed triglyceride accumulation in the livers of rats fed a high-fat diet. Total alkaloids of blackberry roots have previously been shown to alleviate hepatic triglyceride accumulation by reducing mRNA expression of FAS and ACC in rats fed high-fat diets.21,31 However, no previous study had yet determined the effect of blackberry leaf extract on NAFLD. Our previous in vitro study demonstrated that the 50% ethanol extracts of blackberry leaf reduced triglyceride deposition in HepG2 cells better than those of blackberry fruits.23 In addition, the mixture of blackberry leaf and fruit (2:1) extracts inhibited triglyceride accumulation at a lower dosage than that by the individual extracts. The present investigation revealed that BLF (the mixture of blackberry leaf and fruit 2:1) can prevent NAFLD at a lower dosage than BL or BF. Thus, BLF can be a therapeutic candidate for NAFLD.
Triglyceride deposition can be caused by either increased fatty acid synthesis or by decreased oxidation in the liver. In the present study, high-fat diets increased triglyceride deposition, resulting in the development of NAFLD in the NAFLD-control group. The BL, BF, and BLF groups showed a decrease in triglyceride deposition. The decrease may be associated with fatty acid oxidation and the de novo synthesis of fatty acid.7 The mRNA expression fatty acid synthesis-related enzymes, ACC, FAS, and SREBP-1c, increases, and that of the genes involved with fatty acid oxidation-regulatory enzyme, and CPT decrease in NAFLD.7 Disruption in mitochondrial fatty acid oxidation is an important factor in the pathogenesis of NAFLD. Malonyl-CoA synthesized from acetyl CoA by ACC, a regulatory enzyme for fatty acid de novo synthesis, can synthesize palmitate by FAS, which plays an important role in fatty acid synthesis. The present study demonstrated that BLF decreased the mRNA expression of FAS, SREBP-1c, and ACC and it increased that of CPT-1, indicating that BLF enhanced fatty acid utilization in the liver and reduced accumulation. The major components that reduce triglyceride deposition may be ellagic acid and cyanidin 3-glycoside. They have been reported to increase mRNA expressions of ACC and FAS, and reduce that of CPT-1.20,32 Ellagic acid is reported to reduce hepatic triglyceride deposition and oxidative stress with Akt signaling to ameliorate NAFLD.32 Therefore, the improvement of NAFLD symptoms in BLF might be associated with the combination of cyanidin 3-glycoside and ellagic acid.
The gut microbiome becomes involved in the host metabolism by fermenting non-digestible carbohydrates, synthesizing vitamins, producing beneficial compounds, and modulation of the immune system. Dysbiosis, which represents alteration of the composition and activities of gut microbiome leading to a deleterious state of the host metabolism, has been linked to obesity, diabetes, inflammatory bowel disease, and NAFLD.33 Bacteria producing short-chain fatty acids (SCFA) and butyrate-producing bacteria alleviate the symptoms of metabolic disease including NAFLD, and excessive acetate production exacerbates them.34,35 In our previous in vitro study,23 BL and BF changed the production of SCFA: BF increased butyric acid and propionic acid the most, whereas BL increased propionic acid more than that in the NAFLD-control group. BF increased Bifidobacteriales and Coriobacteriales. BL decreased Fibrobacterales compared to the NAFLD-controls. Furthermore, Verrucomicrobiaceae, including Akkermansia, was greatly increased in BL and BFL compared to the NAFLD-control group. One of the major categories of gut bacteria, Akkermansia, that were increased in this study, has been recognized to suppress fat accumulation in the body and to improve glucose regulation.36 Thus, bacteria composition can be modified by BL and BF. The present study demonstrated that BL and BF changed the composition of the gut microbiome, but the changes were not that significant. This might be supported by the difficulty in changing the gut microbiome by probiotics and prebiotics.37 However, minimal changes were also accompanied by changes in intestinal integrity, surface area, and the number of goblet cells. These changes appeared to be associated with a high content of Lactobacillus and Akkermansia in BLF.
The applications of this study may be limited by the inclusion of only a single-gender (male) and we cannot rule out the possibility that the effects seen it this study are gender-specific. Furthermore, using an animal model limits the applicability of this research to human disease, and the results should be confirmed by human clinical trials.
In conclusion, a high dosage of BL and a low dosage of BLF prevented high-fat diet-induced liver disease by ameliorating oxidative stress and inflammation. BLF improved intestinal integrity with increasing Lactobacillus and Akkermansia. BLF might, therefore, be used as a therapeutic intervention for the management of NAFLD. The effective human equivalent dose of BLF is approximately 1.3 g/day.38
Authors’ contributions
SP and HJY participated in designing the study, receiving funding, and writing the manuscript. SMC and BRM conducted biochemical experiments. JYQ and YH conducted the animal study.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by “Food Functionality Evaluation program” under the Ministry of Agriculture, Food and Rural Affairs in 2018 and partly Korea Food Research Institute (E0150302-05).
ORCID iD
Sunmin Park https://orcid.org/0000-0002-6092-8340
References
- 1.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and Meta-analysis. Lancet Gastroenterol Hepatol 2019; 4:389–98 [DOI] [PubMed] [Google Scholar]
- 2.Polyzos SA, Kountouras J, Mantzoros CS. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol 2017; 42:92–108 [DOI] [PubMed] [Google Scholar]
- 3.Ashraf NU, Sheikh TA. Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Free Radic Res 2015; 49:1405–18 [DOI] [PubMed] [Google Scholar]
- 4.Ou Q, Weng Y, Wang S, Zhao Y, Zhang F, Zhou J, Wu X. Silybin alleviates hepatic steatosis and fibrosis in NASH mice by inhibiting oxidative stress and involvement with the Nf-kappaB pathway. Dig Dis Sci 2018; 63:3398–408 [DOI] [PubMed] [Google Scholar]
- 5.Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int 2012; 32:701–11 [DOI] [PubMed] [Google Scholar]
- 6.Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol 2012; 32:2068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol 2014; 7:221–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2:901–10 [DOI] [PubMed] [Google Scholar]
- 9.Engin A. Non-Alcoholic fatty liver disease. Adv Exp Med Biol 2017; 960:443–67 [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Wang Q, Chang R, Zhou X, Xu C. Intestinal barrier function-non-alcoholic fatty liver disease interactions and possible role of gut microbiota. J Agric Food Chem 2019; 67:2754–62 [DOI] [PubMed] [Google Scholar]
- 11.Doulberis M, Kotronis G, Gialamprinou D, Kountouras J, Katsinelos P. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism 2017; 71:182–97 [DOI] [PubMed] [Google Scholar]
- 12.Gkolfakis P, Dimitriadis G, Triantafyllou K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 2015; 14:572–81 [DOI] [PubMed] [Google Scholar]
- 13.Wong T, Wong RJ, Gish RG. Diagnostic and treatment implications of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Gastroenterol Hepatol 2019; 15:83–9 [PMC free article] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty A, Syn WK. Current treatment options for nonalcoholic fatty liver disease. Curr Opin Gastroenterol 2019; 35:168–76 [DOI] [PubMed] [Google Scholar]
- 16.Aghazadeh S, Amini R, Yazdanparast R, Ghaffari SH. Anti-apoptotic and anti-inflammatory effects of silybum marianum in treatment of experimental steatohepatitis. Exp Toxicol Pathol 2011; 63:569–74 [DOI] [PubMed] [Google Scholar]
- 17.Federico A, Dallio M, Loguercio C. Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules 2017; 22:pii:E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filozof C, Goldstein BJ, Williams RN, Sanyal A. Non-Alcoholic steatohepatitis: limited available treatment options but promising drugs in development and recent progress towards a regulatory approval pathway. Drugs 2015; 75:1373–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Zhao J, Zheng Y, Wu J, Liu Y, Peng J, Hong Z. Protective effects and mechanisms of total alkaloids of rubus alceaefolius poir on nonalcoholic fatty liver disease in rats. Mol Med Rep 2014; 10:1758–64 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhao L, Wang D, Huo Y, Ji B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J Sci Food Agric 2016; 96:2494–503 [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Zheng H, Liu Y, Lin J, Zhong X, Xu W, Hong Z, Peng J. Anti-inflammatory effects of total alkaloids from rubus alceifolius poir [corrected]. on non-alcoholic fatty liver disease through regulation of the NF-kappaB pathway. Int J Mol Med 2013; 31:931–7 [DOI] [PubMed] [Google Scholar]
- 22.Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol 2017; 241:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Jin BR, Yang HJ, Kim MJ, S P. A mixture of blackberry leaf and fruit extracts decreases fat deposition in HepG2 cells, modifying the gut microbiome. J Appl Biol Chem 2019; 62:229–237 [Google Scholar]
- 24.Milosevic N, Milanovic M, Abenavoli L, Milic N. Phytotherapy and NAFLD–from goals and challenges to clinical practice. Rev Recent Clin Trials 2014; 9:195–203 [PubMed] [Google Scholar]
- 25.Park S, Kim DS, Kang ES, Kim DB, Kang S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am J Physiol Endocrinol Metab 2018; 315:E99–109 [DOI] [PubMed] [Google Scholar]
- 26.Park S, Kim DS, Wu X, Q JY. Mulberry and dandelion water extracts prevent alcohol-induced steatosis with alleviating gut microbiome dysbiosis. Exp Biol Med 2018; 243:882–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, Zhang T, Qiu JY, Wu X. The combination of mulberry extracts and silk amino acids alleviated high fat diet-induced nonalcoholic hepatic steatosis by improving hepatic insulin signaling and normalizing gut microbiome dysbiosis in rats. Evid Based Complement Alternat Med 2019; 2019:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong L, Huang L, Xue Q, Liu C, Xu K, Shen W, Deng L. Cell-specific elevation of Runx2 promotes hepatic infiltration of macrophages by upregulating MCP-1 in high-fat diet-induced mice NAFLD. J Cell Biochem 2019. Epub ahead of print. doi: 10.1002/jcb.28456 [DOI] [PubMed] [Google Scholar]
- 29.Moon NR, Kang S, Park S. Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-beta1 and Wnt signaling pathways. J Photochem Photobiol B 2018; 178:92–100 [DOI] [PubMed] [Google Scholar]
- 30.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int 2017; 37: 85–9 [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhao J, Zheng H, Zhong X, Zhou J, Hong Z. Treatment of nonalcoholic fatty liver disease with total alkaloids in rubus aleaefolius poir through regulation of fat metabolism. Evid Based Complement Alternat Med 2014; 2014:768540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Hu J, Sheng L, Yuan M, Wu Y, Chen L, Wang G, Qiu Z. Ellagic acid ameliorates AKT-driven hepatic steatosis in mice by suppressing de novo lipogenesis via the AKT/SREBP-1/FASN pathway. Food Funct 2019; 10:3410–20 [DOI] [PubMed] [Google Scholar]
- 33.Aragones G, Gonzalez-Garcia S, Aguilar C, Richart C, Auguet T. Gut Microbiota-Derived mediators as potential markers in nonalcoholic fatty liver disease. Biomed Res Int 2019; 2019:8507583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One 2013; 8:e63388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016; 534:213–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019; 25:1096–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018; 174:1388–405.e21 [DOI] [PubMed] [Google Scholar]
- 38.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharma 2016; 7:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]