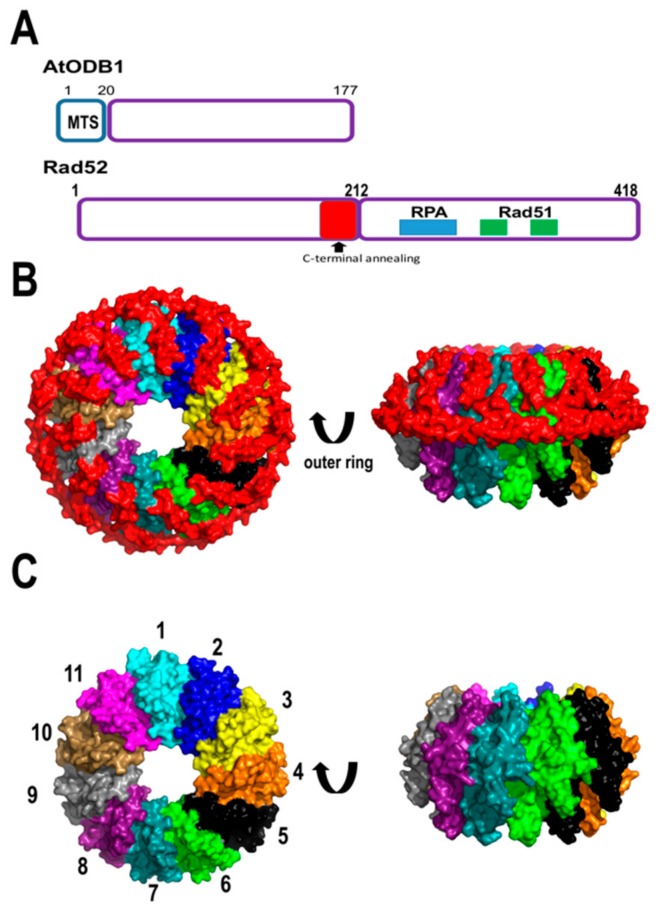
Figure 6.
AtODB1 resembles human Rad52. (A) Structural domain organization of AtODB1 in comparison to human Rad52. AtODB1 lacks the C-terminal domain necessary to interact with RPA and Rad51; (B) crystal structure of the undecameric ring of human Rad52. The undecameric structure is stabilized by alpha-helix 5 that interacts with alpha-helix 1 of the neighbor molecule. Each subunit (residues 1 to 172 is individually colored) and the C-terminal residues (172 to 212) are colored in read. (C) Model of AtODB1 as a undecameric ring lacking alpha-helix 5 of human Rad52.