Abstract
Abiotic stress is one of the major threats affecting plant growth and production. The harm of abiotic stresses includes the disruption of cellular redox homeostasis, reactive oxygen species (ROS) production, and oxidative stress in the plant. Plants have different mechanisms to fight stress, and these mechanisms are responsible for maintaining the required homeostasis in plants. Recently, the study of gasotransmitters in plants has attracted much attention, especially for abiotic stress. In the present review, abiotic stressors were mostly found to induce gasotransmitter production in plants. Meanwhile, these gasotransmitters can enhance the activity of several antioxidant enzymes, alleviate the harmfulness of ROS, and enhance plant tolerance under various stress conditions. In addition, we introduced the interaction of gasotransmitters in plants under abiotic stress. With their promising applications in agriculture, gasotransmitters will be adopted in the near future.
Keywords: gasotransmitters, abiotic stress, production, antioxidant enzyme, interaction
1. Introduction
In nature, plants are constantly challenged by a variety of abiotic stressors, such as heavy metals, low or high temperature, drought or osmotic pressure, salt, and ultraviolet irradiation. Several studies have found that plants are affected in their height, leaf morphology, and stomatal openness under abiotic stress [1,2,3]. In addition, the physiological metabolism of plants is disordered, whereby the contents of proline (Pro), electrolyte leakage (EC), malondialdehyde (MDA), and hydrogen peroxide (H2O2) are changed by abiotic stress [4,5]. At the same time, as the activities of some antioxidant enzymes change, the production of ROS is considered to be a commonplace factor in plants’ responses to abiotic stress [6,7,8]. Researchers have found that the redox environment in a cell is maintained within a “Goldilocks zone”, wherein ROS production is sufficiently counterbalanced by antioxidant capacity and quality control systems [9]. However, when faced with persistent oxidative stress, the redox environment could be pushed outside of this Goldilocks zone, where cell death and damage ensues [9]. Therefore, adjusting the plant’s redox homeostasis is a necessary aspect of abiotic stress resistance. To adapt to such stresses, plants have developed detailed mechanisms to perceive external signals and to embody adaptive responses with suitable physiological and morphological changes [10].
Gasotransmitters are small gas molecules that are generated by organisms and transmit biological signals. Research on gasotransmitters is rapidly expanding and knowledge regarding the potential of gasotransmitters in biology and medicine is accumulating [11]. Gasotransmitters, such as hydrogen gas (H2), hydrogen sulfide (H2S), nitric oxide (NO), carbon monoxide (CO), and methane (CH4), are unique and regulate specific biological functions. Gasotransmitters have long been of great interest in a wide range of fields. Over the past few decades, the roles of these signaling molecules have been extensively studied for their biological applications. Recently, the emissions of endogenous gasotransmitters in plants have been widely studied and analyzed, thereby providing information to facilitate our understanding of new gasotransmitter signaling pathways. Previous studies found that in response to abiotic stressors, plants usually produce these gasotransmitters [12,13,14]. In addition, there is now considerable evidence to show that gasotransmitters can play a pivotal role in enhancing plant tolerance [2,15,16]. Subsequently, biological gases from complex extra and intracellular pathways and gas mediators may regulate many processes in an antagonistic or synergistic way.
In the present review, we introduce the production of gasotransmitters in plants under abiotic stress. Meanwhile, we focused on the recent advances in the roles of gasotransmitters under abiotic stresses and their interaction with other gasotransmitters.
2. Production of Gasotransmitters under Adverse Conditions
2.1. Hydrogen Gas (H2)
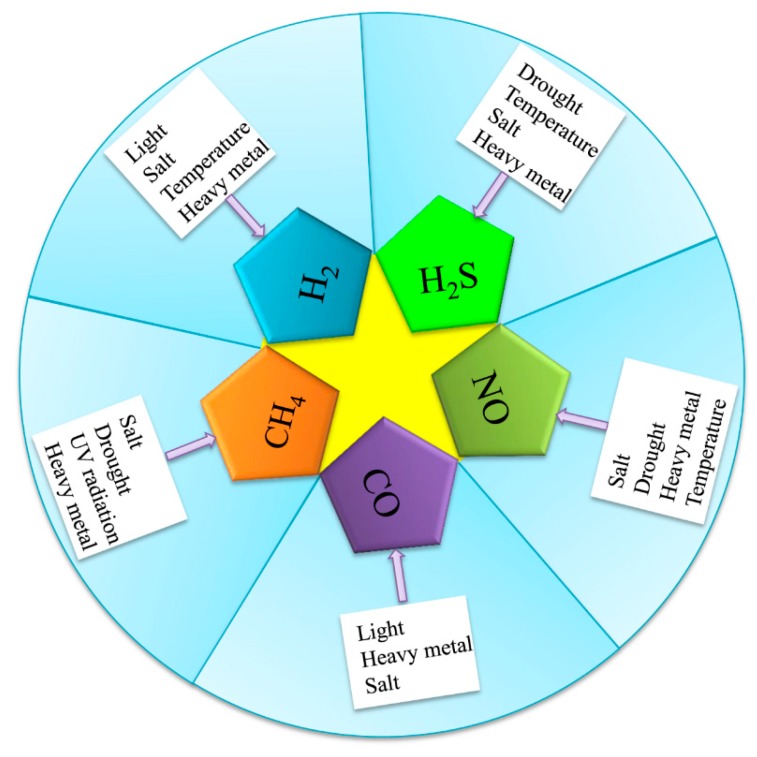
Early in the 20th century, the emissions of H2 were first observed in bacteria by Stephenson and Stickland [17], who found that H2 is produced due to the presence of hydrogenase in bacteria. Subsequent studies showed that H2 could also be produced by green algae and higher plants [18]. Renwick et al. [19] found that lettuce seed germination could release H2 under bright light. Recently, studies have discovered that plants can produce H2 under abiotic stresses. For example, H2 production is induced by salt stress in rice [20] (Figure 1) and alfalfa [21]. Xu et al. [22] also found that H2 is produced in rice under a cold stress stimulation. The exposure of alfalfa to paraquat stress increased endogenous H2 production [23]. Under aluminum stress, rice also produced H2 [16]. Meanwhile, H2 production was found to be induced by abscisic acid, ethylene, and jasmonate acid; salt; and drought stress in rice [24] (Figure 1). However, although some evidence of H2 is produced in plants under abiotic stresses, H2 does not have a clear mechanism of production in plants.
Figure 1.
Multiple environmental stressors can induce gasotransmitter production in plants. Abiotic stressors (drought, salt, heavy metal, temperature, light, and UV radiation) induced the generation of gasotransmitters (H2, H2S, NO, CO, and CH4).
2.2. Hydrogen Sulfide (H2S)
H2S is thought to be a key signalling molecule, and there is growing interest in the roles of H2S in plants [25]. H2S is produced in response to many abiotic stressors, including drought, temperature, and heavy metal stress [26]. Several studies have demonstrated that drought stress induces H2S production in Arabidopsis thaliana [2,27] (Figure 1). Meanwhile, abscisic acid (ABA) application improved the endogenous H2S content in wheat under drought stress [28]. In addition, increasing evidence has indicated that temperature stress also induces the release of H2S in grape [29] and cucumber [30]. Cheng et al. [31] found that H2S generation in poplars is rapidly induced by high temperatures. Shi et al. [32] found that endogenous H2S is evidently induced by cadmium (Cd) stress treatment in Bermuda grass. Lead exposure also induced H2S production in cauliflower [33]. Valivand et al. [34] also reported that nickel (Ni) stress increased H2S content in zucchini. Khan et al. [35] showed that wheat seedlings released H2S under osmotic stress. Glyphosate-induced H2S released from Arabidopsis [36]. Interestingly, Aghdam et al. [37] found that the treatment of hawthorn fruit by exogenous H2S under cold stress can lead to a release of endogenous H2S. Therefore, Jost et al. [38] suggested that H2S is produced from L-cysteine in the presence of hydrogen cyanide, which is catalyzed by ß-cyanoalanine synthase in plants, though the mechanism of production under abiotic stress remains to be further studied.
2.3. Nitric Oxide (NO)
One of the oldest (and still popular) topics in plant NO research is the synthesis of this gaseous molecule [39]. In higher plants, NO can be generated by oxidative and reductive mechanisms involving both enzymatic and nonenzymatic systems [40]. Klepper [41] was the first to observe the production of NO in herbicide-treated soybean. Subsequently, NO was shown to be produced under salt stress in tobacco [42] and Arabidopsis [43] (Figure 1). The authors found that the NO increase in tobacco leaves under stress conditions was due to the induction of nitrate reductase. Thus, NO generation could be closely related to plant nitrate assimilation. Meanwhile, exogenous NO and arginine stimulated the production of endogenous NO in wheat under drought conditions [44]. Liao et al. [45] found that marigold explants also stimulated NO production under drought stress. Exogenous CH4 and sodium nitroprusside triggered the production of NO under osmotic stress [46]. Recently, studies have found that heavy metal aluminum stress induced NO generation in wheat [5,47] and peanut [48]. Moreover, cadmium induced NO production in Arabidopsis [49] and in the lichen Ramalina farinacea [50]. Significantly, NO production was found to be induced by phytohormone and other signaling molecules, such as indole-3-butyric acid [51], 1-methylcyclopropene [52], ABA [53], and hydrogen-rich Water (HRW) [54].
2.4. Carbon Monoxide (CO)
The production of CO in plants was first discovered by Wilks [55]. Subsequently, Tarr et al. [56] reported the direct emission of CO by lima beans exposed to sunlight. Moreover, abiotic stressors may induce CO production in plants. CO production was reported in the roots of Medicgo Sative under cadmium stress [57] (Figure 1). Zilli et al. [58] found that soybean leaves and roots release CO under salt stress. Light induced the release of CO from Arabidopsis by stimulating plant pigment B [14]. Therefore, CO was produced mainly by enhancing heme oxygenase (HO) activity.
2.5. Methane (CH4)
Firstly, Nouchi et al. [59] discovered that CH4 was produced in rice. Then, Keppler et al. [60] reported that rice paddies produced CH4 in aerobic conditions. Meanwhile, CH4 was found under ultraviolet radiation in tobacco [61], as well as in Betula populifolia, Crataegus laevigata, Malus domestica, Plantago lanceolata, Quercus robur, Salix alba, Salix caprea [62], and Brassica oleracea [63]. Brüggemann et al. [64] found that poplar released CH4 under low light conditions. Alfalfa encourages CH4 production under salt stress [65]. Han et al. [66] reported that polyethylene glycol increases CH4 production in maize. Some researchers have demonstrated that alfalfa produces CH4 under heavy metal stress, such as from copper (Cu) [67], aluminum (Al) [13], or cadmium (Ca) [68]. Pea leaves release CH4 at high temperatures [12]. Recently, Martel and Qaderi [69] found that CH4 is also produced in canola under blue light (Figure 1). Interestingly, Messenger et al. [70] found that CH4 is produced in citrus fruit under ultraviolet radiation (UV) when UV reacts with its photosensitizer to produce hydroxy radicals (·OH), which causes the pectin methyl group to form CH4.
3. The Role of Gasotransmitters under Adverse Conditions
3.1. Hydrogen Gas (H2)
3.1.1. Heavy Metal Stress
Heavy metals cause serious environmental pollution across the world, threatening human health and plant growth development. Cd is a toxic metal that can be rapidly absorbed by roots and accumulated in diverse plants, thereby hampering alfalfa and cole seedling growth. Cui et al. [6] observed that hydrogen rich water pretreatment (HRW, which is a safe, economical, and easily available method that provides a valuable approach to investigate the physiological functions of H2 in the scientific field), could alleviate Cd-induced growth inhibition in alfalfa seedlings. The authors found that HRW attenuated Cd toxicity by enhancing antioxidant enzyme activities, including SOD, POD, APX, and enhancing the transcripts levels of relevant antioxidant genes, such as Cu, Zn-SOD, and APX1/2 in the root tissues of alfalfa plants. HRW enhanced the antioxidant capacity of Chinese cabbage under Cd stress and decreased ROS production [8] (Table 1). In addition, glutathione (GSH) is considered to be the main thiol-disulfide redox buffer of the cell. Research has demonstrated that the content of GSH reduction is important in maintaining a redox environment [71]. H2 might be an important regulatory factor in improving the tolerance of Brassica campestris seedlings against Cd, primarily by governing GSH homeostasis [72]. Dai et al. [73] suggested that HRW alleviated Cd toxicity chiefly by reducing oxidative damage promoting sulfur compound metabolism and maintaining nutrient element homeostasis in alfalfa. Al toxicity may also destroy redox homeostasis in plants. Further, 75% HRW pretreatment could significantly mitigate Al toxicity in maize seedlings, chiefly through re-establishing redox homeostasis and maintaining nutrient homeostasis [74]. Meanwhile, Xu et al. [16] observed that HRW mitigates the germination inhibition of rice seeds caused by Al stress, and HRW-regulated miRNA and its target gene expression might be an important reason for this. Additionally, exposure of alfalfa seedlings to Al not only increased NO production but also led to an inhibition of root elongation [75]. However, HRW pretreatment decreased NO production, ultimately alleviating the toxicity of Al in alfalfa seedling roots [75]. Mercury (Hg) stress could also cause oxidative damage to alfalfa, while H2 treatment could reduce the accumulation of Hg in alfalfa seedlings and consequently enhance plant growth upon Hg exposure [76] (Table 1). H2 has been indicated to relieve abiotic stress in cells, thus improving responses to stress challenges in plants [73]. There are two spin states (para- and ortho-) of H2 [77]. Some enzymes involved in ROS metabolism and signaling have been shown to be affected by magnetic fields. The crosstalk between the magnetic field and H2 was suggested to be a possible mechanism for changing cell functions [77]. Therefore, H2 alleviates heavy metal toxicity mainly by decreasing ROS content and enhancing the activities of typical antioxidant enzymes.
Table 1.
H2 involved in plant abiotic stress tolerance.
| Plant Species | Abiotic Stress and Its Effect | H2 Roles under Stress | Reference |
|---|---|---|---|
| Alfalfa | Cd stress inhibited root elongation | Improving root growth, re-establishing glutathione homeostasis | [6] |
| Cabbage | Cd stress reduced the activities of the antioxidant enzyme | Enhancing the activities of the antioxidant enzyme | [8] |
| Cole | Cd stress affected the balance of glutathione | Governing reduced glutathione homeostasis | [72] |
| Alfalfa | Cd stress obviously inhibited alfalfa seedling growth | Attenuating damage in alfalfa seedlings, reducing oxidative damage | [73] |
| Alfalfa | Al stress increased NO production, inhibited root elongation | Improving seedling growth, decreasing NO production | [75] |
| Maize | Al stress inhibited seed germination, broke the ion balance | Alleviating Al toxicity, decreasing lipid peroxidation | [74] |
| Rice | Al stress enhanced oxidative damage | Alleviating germination inhibition, re-establishing redox homeostasis | [16] |
| Alfalfa | Hg stress promoted ROS production | Decreasing ROS production and alleviating oxidative stress | [76] |
| Arabidopsis | Salt stress increased ion outflow | Maintaining ion homeostasis, controlling sodium exclusion | [21] |
| Rice | Salt inhibited seed germination | The alleviation of oxidative damage | [20] |
| Cucumber | Temperature stress affected photosynthetic parameters | Altering photosynthetic gas exchange | [78] |
| Rice | Temperature stress destroyed redox homeostasis | Re-establishing redox homeostasis | [22] |
| Radish | UV-A stress reduced anthocyanin content | Upregulating the anthocyanin biosynthesis-related genes | [79] |
| Alfalfa | UV-B stress destroyed the antioxidant defense system | Reducing lipid peroxidation, regulating the antioxidant defence system | [80] |
| Radish | Short wavelength light stress influenced anthocyanin biosynthesis | Enriching anthocyanin content | [81] |
| Alfalfa | Oxidative stress enhanced oxidative damage | Increasing levels of the MsHO-1 transcript, alleviating oxidative stress | [23] |
| Alfalfa | Drought stress destroyed the redox balance | Modulating stomatal sensitivity, reducing transpirational water loss | [82] |
| Alfalfa | Drought stress affected the enzyme activity | Elevating H2O2 levels, the inhibition of NADPH oxidase | [10] |
3.1.2. Salt and Temperature Stresses
Normally, salinity retards seed germination and inhibits seedling growth, thus significantly reducing crop yields. Sustaining a highly efficient antioxidant system tightly regulated by different groups of transcription factors is important for plants to be able to scavenge salinity-triggered ROS overproduction. In Arabidopsis, H2 (50% HRW) pretreatment modulated the gene and protein expression of the zinc(Zn)-finger transcription factor ZAT10/12 and antioxidant defense-related enzymes, thereby significantly counteracting NaCl-induced ROS excessive production and lipid peroxidation [21] (Table 1). Moreover, H2 also sustained the ion homeostasis of Arabidopsis by regulating the antiporters [21]. Furthermore, HRW enhanced isozymatic activities and the corresponding transcripts of antioxidant enzymes, thus alleviating salt stress in rice during seed germination [20]. Meanwhile, the authors found that the ratio of potassium to sodium in both the shoot and root parts was enhanced by HRW under salt stress [20]. Therefore, H2 might regulate antioxidant systems, Zn-finger transcription factors, and ion homeostasis, thereby enhancing plant salt resistance.
Heat stress is a main limiting factor for plant photosynthesis and membrane stability. Recently, Chen et al. [78] found that H2 is involved in the mitigation of heat-induced oxidative stress by decreasing ROS accumulation, thus enhancing antioxidant enzyme activities and photosynthesis in cucumber seedlings. Additionally, HRW might protect intracellular proteins from heat-induced damage by improving the expression level of heat shock protein 70 [78]. Also, in rice seedlings, genetic evidence has shown that H2 might enhance cold tolerance by re-establishing redox homeostasis through regulating miR398 and miR319 [22] (Table 1). We deduced that the antioxidant enzyme and heat shock proteins play important roles in H2-induced temperature stress resistance.
3.1.3. Ultraviolet Radiation
HRW can enhance UV-A-induced anthocyanin accumulation in radish sprouts and re-establish ROS homeostasis [79] (Table 1). Moreover, a molecular analyses indicated that anthocyanin biosynthesis-related genes were upregulated markedly in radish sprouts by HRW plus UV-A treatment [79]. A similar result was reported for alfalfa in a study by Xie et al. [80], which demonstrated that the biosynthesis of (iso) flavonoids can be enhanced by HRW under UV-B irradiation in alfalfa. In addition, HRW enhanced anthocyanin accumulation, total phenolic content, and alleviated oxidative damage to immature radish microgreens under short wavelength light [81]. Furthermore, HRW might be involved in enhancing key enzyme activities and upregulating of the expression of genes for anthocyanin biosynthesis [81]. These discoveries indicate that H2 acts as a novel cytoprotective promoter of anthocyanin accumulation, enhancing antioxidant enzyme activity and improving plant ultraviolet radiation tolerance.
3.1.4. Drought and Paraquat Stresses
In response to an ABA or water deficit, HRW-pretreated alfalfa seedlings rapidly accumulated higher contents of H2O2 and showed more tolerance to drought stress [65]. Jin et al. [82] found that 50% HRW regulated stomatal closure under drought stress in alfalfa, which was dependent on ABA. The authors also found that HRW could significantly increase the apoplastic potential of hydrogen (pH) of leaves under drought stress. Thus, H2, as a new regulatory mechanism, may enhance alfalfa tolerance to drought stress by elevating H2O2 levels and increasing the apoplastic pH. Additionally, under paraquat-induced oxidative stress, alfalfa treated with HRW showed decreased superoxide anion radical levels and alleviated oxidative stress via heme oxygenase-1 (HO-1; a ubiquitous enzyme catalyzing degradation of heme to produce CO) signalling [23] (Table 1). Therefore, H2 may act as a new bioactive molecule in enhancing plant tolerance to drought and paraquat-induced oxidative stress.
3.2. Hydrogen Sulfide (H2S)
3.2.1. Heavy Metal Stress
The application of an H2S donor (NaHS) enhanced the activities of the ascorbate–glutathione (AsA–GSH) cycle enzymes and decreased the accumulation of ROS, which further maintained the redox status of the cell and mitigated arsenate (As) toxicity in pea [83] (Table 2). H2S maintained Cd and mineral homeostasis in leaves of Cd-stressed foxtail millet [84] and rice [85]. In addition, H2S decreased the EC, MDA, and H2O2 contents, while enhancing photosynthesis in Cd-treated rice seedlings [85]. Meanwhile, under Cd stress conditions, H2S reduced oxidative stress, maintained mineral homeostases, upregulated various antioxidant enzymes, and consequently improved the phenotypic appearance of foxtail millet [84]. Significantly, H2S not only damages foxtail millet by alleviating Cd but also plays a role in reducing the toxicity of Hg. Correspondingly, NaHS (the H2S donor) improved the transcription of bZIP60, a membrane-associated transcription factor, and enhanced the expressions of nonprotein thiols (NPT) and metallothioneins, thereby adequately alleviating Hg toxicity and significantly promoting the growth of rice [4]. Similar results for NaHS, a common donor of H2S, were also reported in cauliflower under lead (Pb) stress condition. H2S elevated NPT and GSH levels to chelate Pb or clean ROS directly, thus enhancing antioxidant enzyme activities and eventually ameliorating seedling germination and growth [33]. Moreover, H2S reduced H2O2 and MDA contents and upregulated calmodulin gene expression in the leaves of Ni-stressed zucchini seedlings [34] (Table 2). Together, H2S may act as an antioxidant to inhibit or clean ROS production to maintain lower MDA and H2O2 levels and improve mineral homeostasis, thereby preventing the oxidative damage of heavy metals in plants.
Table 2.
H2S involved in plant abiotic stress tolerance.
| Plant Species | Abiotic Stress and Its Effect | H2S Roles under Stress | Reference |
|---|---|---|---|
| Rice | Cd stress affected the stability of the membrane | Improving oxidative damage and maintaining ROS homeostasis | [85] |
| Foxtail millet | Cd stress broke the ion balance | Decreasing electrolytic leakage and enhancing photosynthesis | [84] |
| Pea | As stress damaged proteins and membranes | Increasing the level of NO, alleviating oxidative damage | [83] |
| Rice | Hg stress promoted ROS production | Improving the transcription of bZIP60, alleviating Hg toxicity | [4] |
| Cauliflower | Pb stress destroyed GSH levels | Elevating nonprotein thiols and total GSH levels | [33] |
| Zucchini | Ni stress reduced antioxidant enzyme activity | Enhancing antioxidant enzyme activity and reducing Pro contents | [34] |
| Wheat | Salt stress inhibited growth of wheat | Decreasing the Na+ concentration, alleviating the growth inhibition of wheat | [86] |
| Cucumber | Salt stress induced oxidative stress | Maintaining Na+ and K+ homeostasis | [87] |
| Broad bean | Salt stress affected stomatal sensitivity | Inducing stomatal closure, promoting H2O2 production | [88] |
| Cucumber | Salt stress broke the redox balance | Alleviating oxidative damage, upregulating the CsNMAPK transcript level | [89] |
| Grape | Low temperature stress affected the plasma membrane stability | Improving SOD activity and the plasma membrane stability of grape | [29] |
| Banana | Low temperature disrupted ion stability | Maintaining a higher peel firmness, reducing accumulation of MDA | [90] |
| Banana | Low temperature stress broke the redox balance | Inhibiting electrolyte leakage and reducing ethylene production | [91] |
| Hawthorn | Low temperature stress decreased antioxidant enzyme activity | Promoting phenols accumulation and enhancing antioxidant enzyme activity | [37] |
| Cucumber | Low temperature stress influenced the expression of related genes | Upregulating the expression of Cucurbitacin C synthetase-encoding genes | [30] |
| Tobacco | Heat temperature stress decreased vitality of cells | Improving vitality of cells and alleviating electrolyte leakage | [92] |
| Poplar | Heat temperature stress reduced S-nitrosoglutathione reductase activity | Increasing S-nitrosoglutathione reductase activity and reducing reactive oxygen/nitrogen damage | [31] |
| Soybean | Drought stress affected plant photosynthesis | Enhancing chlorophyll contents and decreasing the production of H2O2 | [93] |
| Arabidopsis | Drought stress changed the expression of drought associated genes | Stimulating the expression of drought associated genes | [27] |
| Arabidopsis | Drought stress influenced the transcriptional expression of the ABA receptor | Decreasing transcriptional expression of ABA receptor | [94] |
| Wheat | Drought stress changed MDA contents | Increasing antioxidant enzymes activity and reducing MDA contents | [28] |
| Wheat | Osmotic stress destroyed cysteine homeostasis | Sustaining antioxidant enzymes and cysteine homeostasis | [35] |
3.2.2. Salt Stress
Under salt stress, H2S nonselectively regulates the cation channels and overly sensitive salt pathways by maintaining a lower Na+ concentration, thus alleviating growth inhibition in wheat seedlings [86] (Table 2). The application of H2S could protect cucumber seedlings under salt stress, likely by maintaining Na+/K+ balance, controlling the endogenous H2S levels, and enhancing the antioxidant system under salt stress [87]. Additionally, 0.05 mM NaHS (a donor of H2S) was involved in stomatal closure under salt stress, which may function downstream of H2O2 stomatal movement in the Vicia faba [88]. Interestingly, Qi et al. [89] found that H2S (100 μM NaHS) played a beneficial role in cucumber seedlings under nitrate stress, and mitogen-activated protein kinase (MAPK)/NO signaling was involved in the process by modulating antioxidant enzyme activities (Table 2). We conclude that plants lower stomatal conductance, and reduce the content of water, Na+, and K+ in their tissues under salt conditions. Thus, we suggest that H2S can reduce Na+ concentration, induce stomatal closure, regulate antioxidant enzyme activities, and alleviate the tolerance of plants to salt stress.
3.2.3. Temperature Stress
H2S can regulate the gene expression of VvICE1 and VvCBF3, decrease the contents of the superoxide anion radical and MDA, and enhance superoxide dismutase (SOD) activity and the plasma membrane balance of grape leaves challenged by low temperatures [29] (Table 2). H2S improved phenylalanine ammonia lyase activity, total phenolic contents, and antioxidant capacity, thereby scavenging ROS accumulation and further improving the tolerance of chilling in banana fruit [90]. Notably, H2S (0.5 mM NaHS) maintained higher activities of H+-ATPase, cytochrome C oxidase, and succinate dehydrogenase, which consequently enhanced the energy status and improved the chilling tolerance in banana fruit [90]. Meanwhile, Aghdam et al. [37] showed that H2S also improved the chilling tolerance of hawthorn fruit by increasing antioxidant enzyme activities and promoting phenol accumulation. Thus, these results indicate that H2S can regulate the expression of related genes, improve the activity of antioxidant enzymes, and promote the accumulation of phenolic substances to enhance the tolerance of plants challenged by low temperature stress [91].
With the involvement of Ca2+ and calmodulin, an H2S donor (NaSH) could improve cell vitality, reduce EC, and enhance accumulation of MDA, consequently ameliorating heat tolerance in tobacco suspension cultured cells [92]. Furthermore, H2S reduced S-nitrosoglutathione reductase activity and downstream antioxidant enzyme activities, thereby enhancing tolerance to high temperature stress in poplars [31] (Table 2). The above results highlight a novel signaling mechanism for H2S in plant tolerance to high temperatures and suggest a potential strategy to increase tolerance in plants under high temperature stress by genetically modifying H2S signaling in plants.
3.2.4. Drought Stress
H2S has been reported to reduce the transcriptional expression of ABA receptors in A. thaliana under drought stress conditions, thereby enhancing drought tolerance [94] (Table 2). In addition, the exogenous application of an H2S donor (NaHS) improved ABA biosynthesis and ABA reactivation gene expression levels in wheat leaves and enhanced the plant and relative water content of wheat seedlings leaves [28]. Meanwhile, H2S was found to increase the survival rates of A. thaliana seedlings and significantly reduce the stomatal pore size under drought stress [27]. In a further study, the authors found that H2S could cause a stomatal closure in A. thaliana by regulating the changes in the ion channel activity under drought stress, thus alleviating drought tolerance [2]. Furthermore, Zhang et al. [93] showed that H2S (0.1 mM NaHS) modulates antioxidant enzyme activities, effectively increasing chlorophyll content, reducing the MDA content, and increasing the levels of H2O2 and O2⋅−, thus increasing drought tolerance in soybean seedlings. Notably, Khan et al. [35] found that NO and H2S together clearly enhanced the activities of antioxidant enzymes, thus ameliorating the accumulation of Pro and glycine betaine and protecting wheat seedlings from osmotic stress-induced oxidative stress. Therefore, we conclude that H2S, as a gasotransmitter, activates the defense system and maintains the normal functioning of cellular machinery, thereby improving drought resistance in plants.
3.3. Nitric Oxide (NO)
3.3.1. Heavy Metal Stress
Pharmacological evidence has suggested that NO increases AhHsp70 expression, decreases AhANT expression, and prevents the mitochondrial permeability transition pore from opening, thus improving mitochondrial physiological properties in peanuts under Al stress conditions [48] (Table 3). We have focused on the participation of NO in plant responses to heavy metal stresses, and its relationship with ROS, as well as its possible role as a cytoprotective signal molecule [95]. In addition, the application of the NO donor S-nitrosoglutathione enhanced the antioxidant defense system, decreased ROS and H2O2 contents, guaranteed normal indole-3-acetic acid flow towards the roots, and further enhanced Al resistance in wheat seedlings [5,47]. Furthermore, NO enhanced Cd-tolerance in the lichen Ramalina farinacea by regulating the balance of ROS and improving changes in mineral nutrient content and metabolites [50]. Thus, NO may regulate the expression of related genes and physiological metabolism, thereby enhancing heavy metal resistance in plants.
Table 3.
NO involved in plant abiotic stresses tolerance.
| Plant Species | Abiotic Stress and Its Effect | NO Roles under Stress | Reference |
|---|---|---|---|
| Lichen | Cd stress decreased the content of ionic permeate | Regulating ROS balance, increasing Pro and AsA contents | [50] |
| Peanut | Al stress promoted the production of harmful substances | Upregulating AhHsp70 expression and reducing cytochrome c release | [48] |
| Wheat | Al stress destroyed the antioxidant defense system | Enhancing antioxidant defense, improving H2O2 levels | [5] |
| Wheat | Al stress inhibited auxin flow | Improving the oxidized protein levels, guaranteeing normal indole-3-acetic acid flow | [47] |
| Mangrove | Salt stress induced lipid peroxidation | Reducing hydrogen peroxide content and lipid peroxidation | [96] |
| Arabidopsis | Salt stress broke the ion balance | Downregulating the expression of PIN genes, stabilizing IAA17 | [43] |
| Tobacco | Salt affected the activity of antioxidant enzymes | Enhancing the activity of antioxidant enzymes and H2S levels | [42] |
| Chickpea | Salt stress increased electrolyte Leakage and the levels of osmolytes | Enhancing the biosyntheses of antioxidant enzymes | [97] |
| Mustard | Salt stress accelerated oxidative damage | Regulating oxidative stress and photosynthetic performance | [7] |
| Mustard | Salt stress influenced ion balance | Decreasing electrolytic leakage and K+/Na+ ratio | [98] |
| Pisum sativum L. | Salt stress triggered the membrane lipid peroxidation | Reducing accumulations of ROS and MDA | [99] |
| Jatropha curcas | Salt stress accelerated toxic ion accumulation | Ameliorating oxidative damage and toxic ion accumulation | [100] |
| Wheat | Salt stress reduced biomass production and grain yield | Enhancing physiological and biochemical parameters | [1] |
| Wheat | Temperature stress induced oxidative damage | Enhancing the accumulation of gliadin protein and starch | [101] |
| Cherry | Temperature stress destroyed membrane integrity | Maintaining antioxidant system activity and membrane integrity | [102] |
| Marigold | Drought stress induced carbohydrate and nitrogen accumulation | Increasing chlorophyll content and protein content | [45] |
| Wheat | Osmotic stress created oxidative damage | Enhancing the antioxidant defense system, reducing the methyl-glyoxal content | [44] |
| White clover | Drought stress influenced metabolic regulation and transform | Inducing changes of metabolic profiles | [103] |
| Alfalfa | Drought stress inhibited growth physiological processes | Alleviating loss of water content and embryo axis elongation | [104] |
3.3.2. Salt Stress
NO not only affects plant growth and development but also enhances the salinity adaptation of plants [105]. Chen et al. [96] reported that NO reduces H2O2 accumulation and lipid peroxidation and enhances the content of salt stress-reduced GSH and polyphenols, alleviating the oxidative damage in leaves of Aegiceras corniculatum as a consequence (Table 3). Meanwhile, application of NO regulated oxidative stress and markedly improved the photosynthetic performance of mustard grown under salt stress [7]. Additionally, Ahmad et al. [97] found that NO improved relative leaf water contents, photosynthetic pigment biosynthesis, osmolyte content, and the antioxidative defense system, thus mitigating the adverse effects on chickpea plants caused by high salt stress. Notably, NO might also downregulate the expression of PINFORMED genes, leading to reduced auxin levels, and thus stabilizing IAA17 for the repressed auxin signaling in Arabidopsis [43]. Although NO’s functions in plant salt stressor signalling are now becoming well determined, further research is still needed.
Da Silva et al. [42] reported that NO boosted the enzymatic antioxidant system and improved the nonenzymatic antioxidant GSH under salt stress, thereby regulating the metabolic and physiological changes in tobacco. Evidence suggests that NO enhances the oxidase activity in plants under salt stress [42,98]. The NO donor sodium nitroprusside (100 μM) alleviates salt stress in mustard by improving the growth parameters, photosynthetic traits, and nitrogen metabolism, thereby limiting Na+ accumulation and oxidative stress and enhancing Pro content [98]. In addition, NO improved the salt tolerance of Jatropha curcas during the establishment of seedlings by inducing an effective antioxidant system, thus inhibiting toxic ions and ROS accumulation, alleviating oxidative stress, and activating the antioxidant defense system [100]. Further, the application of sodium nitroprusside (source of NO) significantly mitigated growth inhibition and enhanced the contents of antioxidants and osmoprotectants in salt-challenged Pisum sativum L. [99]. Moreover, the NO donor sodium nitroprusside upregulated antioxidant defense mechanisms and increased ascorbic acid (AsA) contents and total phenolic contents (TPC), thereby effectively increasing the growth and grain yield of wheat under salinity stress [1] (Table 3). Collectively, the accumulating data suggest that NO effectively enhances the growth of plants by upregulating antioxidative defense mechanisms and improving metabolic and physiological changes.
3.3.3. Temperature and Drought Stress
NO (150 µM sodium nitroprusside) improved the quality of wheat grain by increasing the accumulation of gliadin protein and starch and decreasing amylolytic activities under heat stress [101]. However, under low temperature stress, NO (500 μM sodium nitroprusside) lowered EC and MDA accumulation, diminished ROS accumulation, and maintained membrane integrity in cornelian cherry fruits [102] (Table 3). Here, we speculate that NO can regulate plant growth and metabolism by enhancing the activities of antioxidant enzymes and maintaining the integrity of membranes under both heat and low temperature conditions.
Recently, Liao et al. [45] reported that 10 mM NO donor sodium nitroprusside might improve the photosynthetic performance of leaves under drought conditions and alleviate the adverse influence of drought on carbohydrate and nitrogen protection in marigold explants, consequently promoting adventitious rooting. Further, the application of NO significantly improved tricarboxylic acid cycle and antioxidant properties, thus maintaining the redox balance in white clover under water-deficit stress [103]. Meanwhile, NO (0.5 mM sodium nitroprusside) enhanced the antioxidant defense system, upgraded the water status, and decreased oxidative damage and methyl-glyoxal toxicity, thereby increasing drought stress tolerance in wheat seedlings [44]. Under short-term water-deficit stress, the expression of MtGLR genes was inhibited by NO, thereby alleviating the loss of water content and embryo axis elongation in Medicago truncatula seedlings [104]. Therefore, NO is regarded as a critical moderator of plant growth under drought stress by enhancing the antioxidant defense system, maintaining redox balance, and improving the photosynthetic performance of leaves, thereby alleviating the loss of water content.
3.4. Carbon Monoxide (CO)
3.4.1. Heavy Metal Stress
As a signal element, CO (50% CO-saturated aqueous solution) mitigated Cd-induced oxidative damage by regulating GSH and AsA homeostasis in alfalfa roots [57] (Table 4). Similarly, the upregulation of HO-1 gene expression was related to the depletion of GSH in the roots of alfalfa under Cd stress, leading transiently to an improvement in antioxidative capabilities [106]. Additionally, Meng et al. [107] found that the CO-mediated alleviation of Hg toxicity was closely connected with the accumulation of Pro and the reduction of nonprotein thiols in mustard. Meanwhile, the expression of the BnHO gene depressed the generation of O2⋅− and H2O2 and protected Brassica napus L. from oxidative injury under Hg stress [3]. Moreover, 1 µM hemin (the water-soluble CO donor) improved the activity of HO-1 transcriptional expression, reduced the accumulation of Zn and the expression of homeostasis-related genes, and strengthened the Zn tolerance of rice seedlings [108]. Thus, under heavy metal stress conditions, CO could improve oxidative stress by enhancing the activities of antioxidative enzymes and antioxidant metabolism in plants.
Table 4.
CO involved in plant abiotic stress tolerance.
| Plant Species | Abiotic Stress and Its Effect | CO Roles under Stress | Reference |
|---|---|---|---|
| Alfalfa | Cd stress destroyed antioxidation enzymatic activities | Modulating glutathione metabolism | [57] |
| Alfalfa | Cd induced a loss of plasma membrane integrity, lipid peroxidation | Upregulating expression of HO-1 gene | [106] |
| Rapeseed | Hg stress inhibited growth and development | Improving antioxidation capacity and expression of BnHO-1 | [3] |
| Mustard | Hg triggered production of O2⋅− and H2O2, as well as peroxides | Improving antioxidative enzymes, reducing oxidative stress | [107] |
| Rice | Zn stress inhibited root elongation | Downregulating of the expression of homeostasis-related genes | [108] |
| Wheat | Salt stress induced oxidative damage | Enhancing the activities of antioxidant enzymes | [109] |
| Wheat | Salt stress caused oxidative damage | Counteracting lipid peroxidation | [110] |
| Rice | Salt stress inhibited seed germination | Alleviating oxidative damage | [111] |
| Wheat | Salt stress reduced antioxidant enzyme activities | Decreasing of superoxide anion overproduction | [112] |
| Cassia obtusifolia L. | Salt stress lowered chlorophyll concentration | Alleviating oxidative damage, improving membrane permeability | [113] |
| Soybean | Salt stress affected the parameters of lipid peroxidation | Improving lipid peroxidation and ureide metabolism | [58] |
| Wheat | Osmotic stress-induced seed germination inhibition | Increasing in the activities of amylase and antioxidant enzyme | [114] |
| Rice | Drought stress inhibited HO activity | Improving the level of HO-1 gene expression and HO activity | [115] |
| Canola | Temperature stress delayed plant development | Enhancing the expression of BnDHN types gene | [15] |
3.4.2. Salt Stress
The exogenous application of low levels of CO reduced the suppression of seed germination and the damage of seedling leaves in wheat under salt stress by enhancing antioxidant enzyme activities [109] (Table 4). Similarly, CO (1.0 µM hematin), at a low concentration, was able to attenuate the seed germination inhibition under salt stress and counteract the lipid peroxidation in sprouting wheat seeds [110]. Ling et al. [112] found that CO (50% CO aqueous solution) might participate in wheat tolerance against salt stress. Moreover, CO’s moderation of programmed cell death (PCD) and prohibition of root growth were related to the decrease of O2⋅− overproduction, partially through the upregulation of SOD and the downregulation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression. Additionally, Zhang et al. [113] reported that 1 μM hematin (an HO-1 inducer and a putative CO donor) increased the levels of cytosolic osmotic substances and antioxidant enzyme activities and reduced the damage to the photosynthetic system under salinity stress, consequently alleviating the growth of seeds and seedlings in Cassia obtusifolia L. deriving from salinity stress. A similar result was verified in rice. CO could have a significantly positive influence on attenuating the inhibition of rice seed germination and seedling growth promoted by saline stress and reducing oxidative damage by activating antioxidant enzymes [111]. Moreover, the application of CO improved lipid peroxidation and ureide metabolism, thus protecting the soybean nodule nitrogen fixation and assimilation in soybean plants under salinity stress conditions [58] (Table 4). Therefore, we suggest that CO could regulate antioxidant enzymes, lipid peroxidation, and the photosynthetic system, thereby alleviating salinity stress in plants.
3.4.3. Drought and Temperature Stress
CO brought about significant enhancement in the activities of amylase and antioxidant enzymes, which were beneficial to the mitigation of drought-stress-induced wheat seed germination inhibition and lipid peroxidation [114]. In rice plants, the level of HO-1 gene expression and HO activity played a significant role in confirming the process of gibberellin-induced PCD in response to drought stress [115] (Table 4). Thus, the level of HO-1 gene expression, lipid peroxidation, and germination inhibition of plant seeds were improved by CO, thereby enhancing plant tolerance to heavy metals.
Currently, investigations of CO in plant tolerance to temperature stress are scarce. The relative expression of BnDHNs in leaves of Brassica napus seedlings under low temperature treatment was related to the participation of CO [15] (Table 4).
3.5. Methane (CH4)
Recently, 0.39 mM CH4 (methane-rich water) reduced thiobarbituric acid reactive substances (TBARS) content and enhanced amylase activities and total sugar contents upon Cu stress; in this way, cellular redox homeostasis was re-established in alfalfa seedlings [67] (Table 5). Moreover, CH4 partly inhibited Cu-induced Pro production by alternating Pro metabolism [67]. A similar result was confirmed under Al stress; 50% CH4 (methane-rich water) alleviated Al toxicity by decreasing Al accumulation in organic-acid-dependent fashion and recovering redox homeostasis [13]. Meanwhile, 1.3 mM CH4 pretreatment re-established GSH and redox homeostasis to alleviate Cd toxicity [68]. Further molecular evidence suggested that Al-induced oxidative damage is also alleviated by CH4 by regulating antioxidative enzyme activities [13]. Significantly, genetic evidence has demonstrated CH4 alleviates Cd accumulation at least partially through the modulation of heavy metal transporters via miR159 and miR167 [68]. As an important gaseous molecule, CH4 will open a new window into plant resistance to heavy metals and may be applied in phytoremediation processes.
Table 5.
CH4 involved in plant abiotic stress tolerance.
| Plant Species | Abiotic Stress and Its Effect | CH4 Roles under Stress | Reference |
|---|---|---|---|
| Alfalfa | Al stress influenced the physiological roles of alfalfa | Enhancing resistance seedlings, regulating organic acid metabolism | [13] |
| Alfalfa | Cu-triggered oxidative stress | Increasing amylase activities, reducing Cu accumulation | [67] |
| Alfalfa | Cd stress decreased the ratio of reduced/oxidized (homo)glutathione | Re-establishing glutathione homeostasis, reducing lipid peroxidation | [68] |
| Alfalfa | Salt reduced the activities of representative antioxidant enzymes | Reducing reactive oxygen species over accumulation | [65] |
| Maize | Osmotic stress decreased biomass and relative water contents | Modulating sugar and AsA metabolism | [66] |
| Mung bean | Osmotic stress broke the ion balance | Re-establishing redox balance, alleviating seed germination inhibition | [46] |
Zhu et al. [65] found that 50% MRW reduced NaCl-induced lipid peroxidation and ROS overaccumulation in alfalfa, and ion homeostasis was re-established. In addition, MRW alleviated the NaCl-induced inhibition of seed germination and oxidative damage, partially by the upregulation of HO-1 [65]. These results could extend our knowledge of CH4 in plants and are also crucial to fundamental plant biology.
Under osmotic stress, not only did the sugar content in maize root tissues increase by CH4 (exogenously applied 0.65 mM), but the sugar and AsA metabolism in maize seedlings were also regulated by CH4 [66]. Further research found a positive role of endogenous NO in CH4-enhanced plant tolerance against osmotic stress in mung beans [46]. This research also suggested that NO-regulated redox homeostasis and S-nitrosylation might take part in the above CH4 action (Table 5). Thus, CH4 can be expected to play an advantageous role in plant tolerance against osmotic stress.
4. Gasotransmitter Interactions under Adverse Conditions
The crosstalk among gasotransmitters was first discovered in animals. However, in recent years, their interaction has also been confirmed in plants under abiotic stress conditions.
4.1. Interaction between H2 and NO
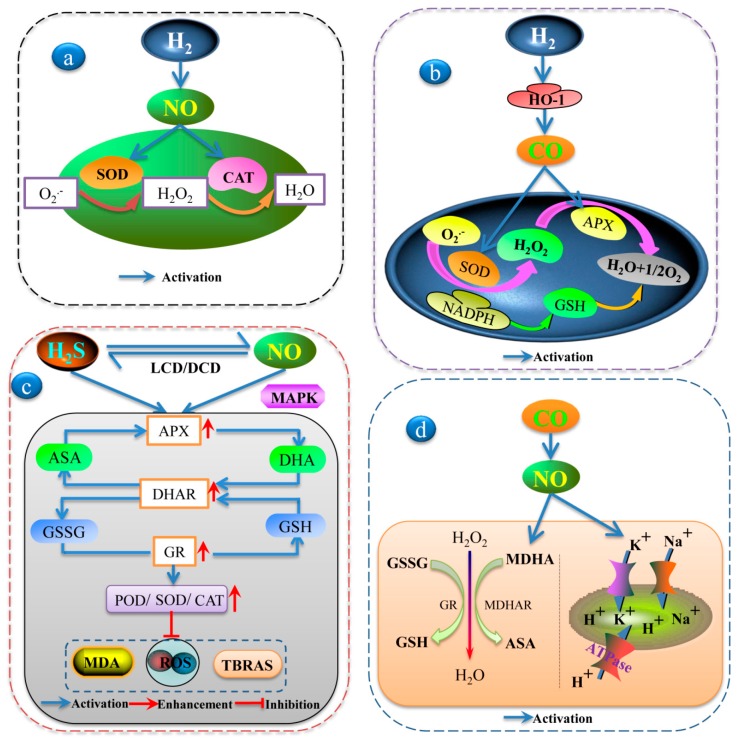
Studies on the mechanisms for H2 signaling in plants are fragmented, although rapid progress is being made this field. According to recent reports, H2 and NO are closely related and alleviate plant tolerance under abiotic stress. NO may play a part downstream in the H2 signaling cascade in plants in response to abiotic stressors, such as heavy metal stress [75] and drought stress [116]. Under drought stress conditions, the addition of H2 and NO could enhance the antioxidant defense system in stressed plants by reducing ROS production and membrane peroxide and upregulating some antioxidant enzyme activities such as SOD, catalase (CAT), and ascorbate peroxidase (APX) [116] (Figure 2a). In addition, Chen et al. [75] reported that the functional interaction of H2 and NO could alleviate Al toxicity symptoms. Our laboratory studies have found that H2 and NO are involved in the growth of adventitious roots. H2 enhanced NO content by upregulating nitrate reductase activities in cucumber explants [54]. Meanwhile, H2 activated the cell cycle and upregulated cell-cycle-related genes and target genes related to adventitious roots via the NO pathway [117]. However, the physiological interaction between NO and H2 is more complex in plants. More studies need to be done in the future to clarify this intricate relationship.
Figure 2.
Schematic model of the interaction among H2, H2S, NO, CO, and CH4 in different abiotic stress processes: (a) The interaction of H2 and NO increased antioxidant defenses in stressed plants. (b) Involvement of HO-1 in H2 induced different environment stress tolerances in plants. (c) The crosstalk between H2S and NO upregulated the ASA-GSH cycle, which enhanced the activity of some antioxidant enzymes and alleviated the damage of abiotic stresses to plants. (d) CO enhanced abiotic stress tolerance via NO-mediated maintenance of ion balance and the upregulation of antioxidant defense in plants.
4.2. Interaction between H2 and CO
The importance of the HO-1/CO signalling system in conferring a tolerance of oxidative damage to plants has been well proven. Jin et al. [82] executed a series of physiological and biochemical experiments to indicate the mechanistic depiction of H2O2 and HO-1 in the H2 signalling of alfalfa seedlings exposed to osmotic stress. NADPH oxidase could be the potential source of H2-induced H2O2 generation. The inhibition of NADPH oxidase and the chemical scavenging of H2O2 could block H2-induced HO-1 expression. Additionally, the interaction between H2 and CO enhanced SOD, peroxidase (POD), and APX activities, and increased well-known antioxidant GSH contents, thereby improving the antioxidant systems in alfalfa when exposed to paraquat-stressors [23] (Figure 2b). Therefore, it is conceivable that H2 can interact with CO as a messenger in plants.
4.3. Crosstalk between H2S and NO
Along with NO and ROS, H2S is involved in numerous stressor responses, including heavy metals, salt, and temperature [26]. H2S signal transduction pathways do not always work independently and are closely connected with NO. The two gases share many collaborative downstream signaling pathways and have some similar functions. When it comes to NO downstream of H2S, Singh et al. [83] found that H2S and NO might both participate in reducing the accumulation of As and triggering the upregulation of the AsA–GSH cycle to counterbalance ROS-mediated damage to macromolecules. Thus, under abiotic stress, NO downstream of H2S not only upregulates the AsA–GSH cycle but also alleviates the oxidative damage of plants through signal transduction. MAPK and NO were essential for abiotic stress signaling. The MAPK inhibitor PD98059 and NO scavengers reversed the alleviating effect of H2S by enhancing MDA and H2O2 content and decreasing the antioxidant enzyme activities of SOD, CAT, POD, and APX, as well as the endogenous H2S contents and L-cysteine desulfhydrase (LCD) activities under nitrate stress [89]. Obviously, H2S primarily affects NO metabolism under environmental stimulations, and H2S’s protective role is likely caused by this effect [25] (Figure 2c).
Conversely, when H2S was downstream, NO markedly increased the activities of glutathione reductase (GR), APX, POD, SOD, and CAT by enhancing the activities of the H2S-synthesizing enzymes LCD and D-cysteine desulfhydrase (DCD), thus alleviating osmotic stress in wheat seedlings [35]. Many redox couples in a cell work together to maintain the redox environment [71], and the GSSG/2GSH couple was one of the principal factors in maintaining cellular redox homeostasis. NO might be located upstream of H2S in Bermuda grass’s response to Cd stress by regulating antioxidant enzyme activities (SOD, CAT, POD, and GR) and the nonenzymatic GSH redox state, thus keeping MDA and cell damage at relatively low levels and enhancing Cd tolerance [32] (Figure 2c). NO and H2S, which mediate various signaling networks, are crucial elements in the biochemistry and physiology of plants [118]. Together, the synergistic or antagonistic effects of H2S and NO might play important roles in the regulation of abiotic stress.
4.4. Crosstalk between NO and CO
NO alleviated the harmfulness of ROS, reacted with the CO molecule, and regulated the activation of the antioxidant enzyme system under various stress conditions. Under salinity stress conditions, Xie et al. [119] reported that CO, as well as NO, obviously upregulated the H+-pump and the activation of CAT, SOD, APX, GR, and dehydroascorbate reductase (DHAR) or their related transcripts, thereby resulting in the enhancement of the K/Na ratio and the alleviation of ROS in wheat (Figure 2d). In this way, CO could confer an increased tolerance to salt stress by maintaining ion homeostasis and improving the antioxidant system parameters in wheat, both of which were partially mediated by the NO signal. Interestingly, NO also plays a regulatory role in HO/CO systems. HO activity was markedly enhanced by NO and indicated a positive correlation with HO-1 transcript levels. Thus, NO may participate in the UV-B-specific signaling pathway that mediates the HO response under low levels of radiation [120]. We speculate that either NO or CO is located downstream in the form of signal transduction and can play a beneficial role in plant growth under abiotic stressors.
4.5. Interaction between CH4 and Other Signallings
Maintaining redox homeostasis is a vital mechanism for maintaining plant tolerance against various stressors. NO regulated the ion balance and sugar breakdown in the CH4 signaling cascade by reducing ROS production in mung beans under osmotic stress [46]. Notably, HO-1/CO and Ca2+ were reported as the downstream signals in CH4-induced cucumber adventitious root formation [121]. Both CH4 and HO-1 could upregulate the expression of the HO-1 gene, thereby enhancing the total or isozymatic activities of other antioxidant enzymes, including APX, SOD, and POD, and further alleviating the growth inhibition of alfalfa seeds under salt stress [65]. Therefore, we conjecture that CH4 may enhance the tolerance of plants via the upregulation of HO-1 under adverse conditions. Additionally, the crosstalk between CH4 and other signals in plant tolerance against abiotic stressors remains rare.
5. Conclusions and Future Perspectives
Over the years, gasotransmitters, including H2, H2S, NO, CO, and CH4, have become a hot issue in the research of abiotic stress, and there has been major work done in this area. Presently, the available reports suggest that these gasotransmitters are released in plants under different adverse conditions. Importantly, these gasotransmitters enhance plant tolerance to a variety of environmental stimulations, mainly by regulating the activity of antioxidant enzymes, mitigating oxidative stress and lipid pexoxidation, maintaining ion homeostasis, and re-establishing GSH homeostasis. In addition, interactions among gasotransmitters have been confirmed in plants under adverse conditions.
Although a growing body of studies shows that plants may produce gasotransmitters under abiotic stresses, future studies on the biosynthesis of these gasotransmitters should focus on the molecular details of their production pathways in plants. The intricate mechanisms associated with their responses to abiotic stimuli are still a subject of great interest. Therefore, the future study of gasotransmitters in plants should concentrate on their molecular mechanisms and their interactions with each other under abiotic stress. Another question remains: “what are the receptors of these gasotransmitters in plants?” So far, proteomics research has shown that NO has three post-translational modifications to the target protein: metal nitration, tyrosine nitration, and S-nitrosation [122]. Additionally, H2S may directly modify protein thiol groups [26], and the thiol group in proteins influence cellular function. However, the target proteins of other gasotransmitters remain unclear. Also, these gasotransmitters are indispensable for plant resistance, and more research should be devoted to field experiments with the aim of enhancing agriculture in the areas of yield and quality.
Abbreviations
| H2 | hydrogen gas |
| H2S | hydrogen Sulfide |
| NO | nitric Oxide |
| CO | carbon Monoxide |
| CH4 | methane |
| EC | electrolyte leakage |
| H2O2 | hydrogen peroxide |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| ABA | abscisic acid |
| GSH | glutathione |
| AsA-GSH | ascorbate–glutathione |
| AsA | ascorbic acid |
| HO-1 | heme oxygenase-1 |
| HO | heme oxygenase |
| HRW | hydrogen-rich water |
| Cu | copper |
| Cd | cadmium |
| Al | aluminum |
| Hg | mercury |
| Zn | zinc |
| Ni | nickel |
| Pb | lead |
| As | arsenate |
| UV | ultraviolet radiation |
| NPT | nonprotein thiols |
| Pro | proline |
| pH | potential of hydrogen |
| SOD | superoxide dismutase |
| MAPK | mitogen-activated protein kinase |
| TPC | total phenolic contents |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| TBARS | thiobarbituric acid reactive substances |
| CAT | catalase |
| APX | ascorbate peroxidase |
| POD | peroxidase |
| LCD | L-cysteine desulfhydrase |
| DCD | D-cysteine desulfhydrase |
| GR | glutathione reductase |
| DHAR | dehydroascorbate reductase |
| PCD | programmed cell death |
| O2⋅− | superoxide anion |
| ·OH | hydroxy radicals |
Author Contributions
All authors listed have made substantial, direct, and intellectual contributions to the work, and approved it for publication.
Funding
This research was funded by the National Key Research and Development Program (grant number 2018YFD1000800); the National Natural Science Foundation of China (grant number 31860568, 31560563, and 31160398); the Research Fund of Higher Education of Gansu, China (grant number 2018C-14); the Post-Doctoral Foundation of China (grant number 20100470887 and 2012T50828); and the Natural Science Foundation of Gansu Province, China (grant number 1606RJZA073 and 1606RJZA077).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ali Q., Daud M., Haider M.Z., Ali S., Rizwan M., Aslam N., Noman A., Iqbal N., Shahzad F., Deeba F. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017;119:50–58. doi: 10.1016/j.plaphy.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Jin Z., Wang Z., Ma Q., Sun L., Zhang L., Liu Z., Liu D., Hao X., Pei Y. Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil. 2017;419:141–152. doi: 10.1007/s11104-017-3335-5. [DOI] [Google Scholar]
- 3.Shen Q., Jiang M., Li H., Che L.L., Yang Z.M. Expression of a Brassica napus heme oxygenase confers plant tolerance to mercury toxicity. Plant Cell Environ. 2011;34:752–763. doi: 10.1111/j.1365-3040.2011.02279.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z., Chen M., Jiang M. Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol. Biochem. 2017;111:179–192. doi: 10.1016/j.plaphy.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Sun C., Liu L., Lu L., Jin C., Lin X. Nitric oxide acts downstream of hydrogen peroxide in regulating aluminum-induced antioxidant defense that enhances aluminum resistance in wheat seedlings. Environ. Exp. Bot. 2018;145:95–103. doi: 10.1016/j.envexpbot.2017.10.020. [DOI] [Google Scholar]
- 6.Cui W., Gao C., Fang P., Lin G., Shen W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013;260:715–724. doi: 10.1016/j.jhazmat.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Fatma M., Masood A., Per T.S., Khan N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016;7:521. doi: 10.3389/fpls.2016.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q., Su N., Cai J., Shen Z., Cui J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 2015;175:174–182. doi: 10.1016/j.jplph.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Alleman R.J., Katunga L.A., Nelson M.A., Brown D.A., Anderson E.J. The “Goldilocks Zone” from a redox perspective—Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 2014;5:358. doi: 10.3389/fphys.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Q., Cui W., Dai C., Zhu K., Zhang J., Wang R., La H., Li X., Shen W. Involvement of hydrogen peroxide and heme oxygenase-1 in hydrogen gas-induced osmotic stress tolerance in alfalfa. Plant Growth Regul. 2016;80:215–223. doi: 10.1007/s10725-016-0159-x. [DOI] [Google Scholar]
- 11.Wang R. Overview of Gasotransmitters and the Related Signaling Network. In: Wang R., editor. Gasotransmitters. Royal Society of Chemistry; Cambridge, UK: 2018. pp. 1–28. [Google Scholar]
- 12.Abdulmajeed A.M., Derby S.R., Strickland S.K., Qaderi M.M. Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system. J. Photochem. Photobiol. B Biol. 2017;166:193–201. doi: 10.1016/j.jphotobiol.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Cui W., Cao H., Yao P., Pan J., Gu Q., Xu S., Wang R., Ouyang Z., Wang Q., Shen W. Methane enhances aluminum resistance in alfalfa seedlings by reducing aluminum accumulation and reestablishing redox homeostasis. Biometals. 2017;30:719–732. doi: 10.1007/s10534-017-0040-z. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y., Li R., Yang W., Chen Z., Hu X. Carbon monoxide signal regulates light-initiated seed germination by suppressing SOM expression. Plant Sci. 2018;272:88–98. doi: 10.1016/j.plantsci.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Maryan K.E., Lahiji H.S., Farrokhi N., Komeleh H.H. Analysis of Brassica napus dehydrins and their Co-Expression regulatory networks in relation to cold stress. Gene Expr. Patterns. 2019;31:7–17. doi: 10.1016/j.gep.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Xu D., Cao H., Fang W., Pan J., Chen J., Zhang J., Shen W. Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: A case study on germination. Ecotoxicol. Environ. Saf. 2017;145:303. doi: 10.1016/j.ecoenv.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson M., Stickland L.H. Hydrogenase: A bacterial enzyme activating molecular hydrogen: The properties of the enzyme. Biochem. J. 1931;25:205. doi: 10.1042/bj0250205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanadze G. Absorption of molecular hydrogen by green leaves in light. Fiziol Rast. 1961;8:555–559. [Google Scholar]
- 19.Renwick G., Giumarro C., Siegel S. Hydrogen metabolism in higher plants. Plant Physiol. 1964;39:303. doi: 10.1104/pp.39.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S., Zhu S., Jiang Y., Wang N., Wang R., Shen W., Yang J. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil. 2013;370:47–57. doi: 10.1007/s11104-013-1614-3. [DOI] [Google Scholar]
- 21.Xie Y., Mao Y., Lai D., Zhang W., Shen W. H2 Enhances Arabidopsis Salt Tolerance by Manipulating ZAT10/12-Mediated Antioxidant Defence and Controlling Sodium Exclusion. PLoS ONE. 2012;7:e49800. doi: 10.1371/journal.pone.0049800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S., Jiang Y., Cui W., Jin Q., Zhang Y., Bu D., Fu J., Wang R., Zhou F., Shen W. Hydrogen enhances adaptation of rice seedlings to cold stress via the reestablishment of redox homeostasis mediated by miRNA expression. Plant Soil. 2017;414:53–67. doi: 10.1007/s11104-016-3106-8. [DOI] [Google Scholar]
- 23.Jin Q., Zhu K., Cui W., Xie Y., Han B., Shen W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2013;36:956–969. doi: 10.1111/pce.12029. [DOI] [PubMed] [Google Scholar]
- 24.Zeng J., Zhang M., Sun X. Molecular Hydrogen Is Involved in Phytohormone Signaling and Stress Responses in Plants. PLoS ONE. 2013;8:e71038. doi: 10.1371/journal.pone.0071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock J.T., Whiteman M. Hydrogen sulfide and cell signaling: Team player or referee? Plant Physiol. Biochem. 2014;78:37–42. doi: 10.1016/j.plaphy.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Hancock J.T. Hydrogen sulfide and environmental stresses. Environ. Exp. Bot. 2019;161:50–56. doi: 10.1016/j.envexpbot.2018.08.034. [DOI] [Google Scholar]
- 27.Jin Z., Shen J., Qiao Z., Yang G., Wang R., Pei Y. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011;414:481–486. doi: 10.1016/j.bbrc.2011.09.090. [DOI] [PubMed] [Google Scholar]
- 28.Ma D., Ding H., Wang C., Qin H., Han Q., Hou J., Lu H., Xie Y., Guo T. Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE. 2016;11:e0163082. doi: 10.1371/journal.pone.0163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu P., Wang W., Hou L., Liu X. Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc. Bot. Pol. 2013;82:295–302. doi: 10.5586/asbp.2013.031. [DOI] [Google Scholar]
- 30.Liu Z., Li Y., Cao C., Liang S., Ma Y., Liu X., Pei Y. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol. Biol. 2019;99:535–544. doi: 10.1007/s11103-019-00834-w. [DOI] [PubMed] [Google Scholar]
- 31.Cheng T., Shi J., Dong Y., Ma Y., Peng Y., Hu X., Chen J. Hydrogen sulfide enhances poplar tolerance to high-temperature stress by increasing S-nitrosoglutathione reductase (GSNOR) activity and reducing reactive oxygen/nitrogen damage. Plant Growth Regul. 2018;84:11–23. doi: 10.1007/s10725-017-0316-x. [DOI] [Google Scholar]
- 32.Shi H., Ye T., Chan Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.) Plant Physiol. Biochem. 2014;74:99–107. doi: 10.1016/j.plaphy.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z., Yang B., Hao Z., Zhu J., Zhang Y., Xu T. Exogenous hydrogen sulfide ameliorates seed germination and seedling growth of cauliflower under lead stress and its antioxidant role. J. Plant Growth Regul. 2018;37:5–15. doi: 10.1007/s00344-017-9704-8. [DOI] [Google Scholar]
- 34.Valivand M., Amooaghaie R., Ahadi A. Interplay between hydrogen sulfide and calcium/calmodulin enhances systemic acquired acclimation and antioxidative defense against nickel toxicity in zucchini. Environ. Exp. Bot. 2019;158:40–50. doi: 10.1016/j.envexpbot.2018.11.006. [DOI] [Google Scholar]
- 35.Khan M.N., Mobin M., Abbas Z.K., Siddiqui M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide. 2017;68:91–102. doi: 10.1016/j.niox.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Corpas F.J., Barroso J.B., González-Gordo S., Muñoz-Vargas M.A., Palma J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Biol. 2019 doi: 10.1111/jipb.12779. [DOI] [PubMed] [Google Scholar]
- 37.Aghdam M.S., Mahmoudi R., Razavi F., Rabiei V., Soleimani A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci. Hortic. 2018;238:264–271. doi: 10.1016/j.scienta.2018.04.063. [DOI] [Google Scholar]
- 38.Jost R., Berkowitz O., Wirtz M., Hopkins L., Hawkesford M., Hell R. Genomic and functional characterization of the oas gene family encoding O-acetylserine (thiol) lyases, enzymes catalyzing the final step in cysteine biosynthesis in Arabidopsis thaliana. Gene. 2000;253:237–247. doi: 10.1016/S0378-1119(00)00261-4. [DOI] [PubMed] [Google Scholar]
- 39.Astier J., Gross I., Durner J. Nitric oxide production in plants: An update. J. Exp. Bot. 2017;69:3401–3411. doi: 10.1093/jxb/erx420. [DOI] [PubMed] [Google Scholar]
- 40.Kolbert Z., Feigl G., Freschi L., Poór P. Gasotransmitters in action: Nitric oxide-ethylene crosstalk during plant growth and abiotic stress responses. Antioxidants. 2019;8:167. doi: 10.3390/antiox8060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klepper L. Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide-treated soybean plants. Atmos. Environ. (1967) 1979;13:537–542. doi: 10.1016/0004-6981(79)90148-3. [DOI] [Google Scholar]
- 42.Da Silva C.J., Fontes E.P.B., Modolo L.V. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci. 2017;256:148–159. doi: 10.1016/j.plantsci.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Liu W., Li R.-J., Han T.-T., Cai W., Fu Z.-W., Lu Y.-T. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015;168:343–356. doi: 10.1104/pp.15.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasanuzzaman M., Nahar K., Rahman A., Inafuku M., Oku H., Fujita M. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol. Mol. Biol. Plants. 2018;24:993–1004. doi: 10.1007/s12298-018-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao W.-B., Huang G.-B., Yu J.-H., Zhang M.-L. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biochem. 2012;58:6–15. doi: 10.1016/j.plaphy.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Su J., Cheng D., Wang R., Mei Y., Hu H., Shen W., Zhang Y. Nitric oxide contributes to methane-induced osmotic stress tolerance in mung bean. BMC Plant Biol. 2018;18:207. doi: 10.1186/s12870-018-1426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faria-Lopes R.O., Muniz D.R., Chaves I.S., França M.G., Modolo L.V. Nitric oxide precursors prevent Al-triggered auxin flow inhibition in Triticum aestivum roots. J. Adv. Res. 2019;15:27–36. doi: 10.1016/j.jare.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He H., Huang W., Oo T.L., Gu M., Zhan J., Wang A., He L.-F. Nitric oxide suppresses aluminum-induced programmed cell death in peanut (Arachis hypoganea L.) root tips by improving mitochondrial physiological properties. Nitric Oxide. 2018;74:47–55. doi: 10.1016/j.niox.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Han B., Yang Z., Xie Y., Nie L., Cui J., Shen W. Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol. Plant. 2014;7:388–403. doi: 10.1093/mp/sst122. [DOI] [PubMed] [Google Scholar]
- 50.Kováčik J., Dresler S., Micalizzi G., Babula P., Hladký J., Mondello L. Nitric oxide affects cadmium-induced changes in the lichen Ramalina farinacea. Nitric Oxide. 2019;83:11–18. doi: 10.1016/j.niox.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Liao W., Huang G., Yu J., Zhang M., Shi X. Nitric oxide and hydrogen peroxide are involved in indole-3-butyric acid-induced adventitious root development in marigold. J. Hortic. Sci. Biotechnol. 2011;86:159–165. doi: 10.1080/14620316.2011.11512742. [DOI] [Google Scholar]
- 52.Liao W.-B., Zhang M.-L., Yu J.-H. Role of nitric oxide in delaying senescence of cut rose flowers and its interaction with ethylene. Sci. Hortic. 2013;155:30–38. doi: 10.1016/j.scienta.2013.03.005. [DOI] [Google Scholar]
- 53.Neill S., Barros R., Bright J., Desikan R., Hancock J., Harrison J., Morris P., Ribeiro D., Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y., Liao W., Wang M., Niu L., Xu Q., Jin X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016;195:50–58. doi: 10.1016/j.jplph.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 55.Wilks S.S. Carbon monoxide in green plants. Science. 1959;129:964–966. doi: 10.1126/science.129.3354.964. [DOI] [PubMed] [Google Scholar]
- 56.Tarr M.A., Miller W.L., Zepp R.G. Direct carbon monoxide photoproduction from plant matter. J. Geophys. Res. Atmos. 1995;100:11403–11413. doi: 10.1029/94JD03324. [DOI] [Google Scholar]
- 57.Han Y., Zhang J., Chen X., Gao Z., Xuan W., Xu S., Ding X., Shen W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2008;177:155–166. doi: 10.1111/j.1469-8137.2007.02251.x. [DOI] [PubMed] [Google Scholar]
- 58.Zilli C.G., Santa-Cruz D.M., Balestrasse K.B. Heme oxygenase-independent endogenous production of Carbon Monoxide by soybean plants subjected to salt stress. Environ. Exp. Bot. 2014;102:11–16. doi: 10.1016/j.envexpbot.2014.01.012. [DOI] [Google Scholar]
- 59.Nouchi I., Mariko S., Aoki K. Mechanism of methane transport from the rhizosphere to the atmosphere through rice plants. Plant Physiol. 1990;94:59–66. doi: 10.1104/pp.94.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keppler F., Hamilton J.T., Braß M., Röckmann T. Methane emissions from terrestrial plants under aerobic conditions. Nature. 2006;439:187. doi: 10.1038/nature04420. [DOI] [PubMed] [Google Scholar]
- 61.McLeod A.R., Fry S.C., Loake G.J., Messenger D.J., Reay D.S., Smith K.A., Yun B.W. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 2008;180:124–132. doi: 10.1111/j.1469-8137.2008.02571.x. [DOI] [PubMed] [Google Scholar]
- 62.Bruhn D., Mikkelsen T.N., Øbro J., Willats W.G.T., Ambus P. Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biol. 2009;11:43–48. doi: 10.1111/j.1438-8677.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 63.Bruhn D., Mikkelsen T.N., Rolsted M.M.M., Egsgaard H., Ambus P. Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biol. 2014;16:512–516. doi: 10.1111/plb.12137. [DOI] [PubMed] [Google Scholar]
- 64.Brüggemann N., Meier R., Steigner D., Zimmer I., Louis S., Schnitzler J.P. Nonmicrobial aerobic methane emission from poplar shoot cultures under low-light conditions. New Phytol. 2009;182:912–918. doi: 10.1111/j.1469-8137.2009.02797.x. [DOI] [PubMed] [Google Scholar]
- 65.Zhu K., Cui W., Dai C., Wu M., Zhang J., Zhang Y., Xie Y., Shen W. Methane-rich water alleviates NaCl toxicity during alfalfa seed germination. Environ. Exp. Bot. 2016;129:37–47. doi: 10.1016/j.envexpbot.2015.11.013. [DOI] [Google Scholar]
- 66.Han B., Duan X., Wang Y., Zhu K., Zhang J., Wang R., Hu H., Qi F., Pan J., Yan Y. Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci. Rep. 2017;7:46185. doi: 10.1038/srep46185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samma M.K., Zhou H., Cui W., Zhu K., Zhang J., Shen W. Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa. Biometals. 2017;30:97–111. doi: 10.1007/s10534-017-9989-x. [DOI] [PubMed] [Google Scholar]
- 68.Gu Q., Chen Z., Cui W., Zhang Y., Hu H., Yu X., Wang Q., Shen W. Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol. Environ. Saf. 2018;147:861–871. doi: 10.1016/j.ecoenv.2017.09.054. [DOI] [PubMed] [Google Scholar]
- 69.Martel A.B., Qaderi M.M. Unravelling the effects of blue light on aerobic methane emissions from canola. J. Plant Physiol. 2019;233:12–19. doi: 10.1016/j.jplph.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Messenger D.J., McLeod A.R., Fry S.C. The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ. 2009;32:1–9. doi: 10.1111/j.1365-3040.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- 71.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 72.Wu Q., Su N., Chen Q., Shen W., Shen Z., Xia Y., Cui J. Cadmium-induced hydrogen accumulation is involved in cadmium tolerance in Brassica campestris by reestablishment of reduced glutathione homeostasis. PLoS ONE. 2015;10:e0139956. doi: 10.1371/journal.pone.0139956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dai C., Cui W., Pan J., Xie Y., Wang J., Shen W. Proteomic analysis provides insights into the molecular bases of hydrogen gas-induced cadmium resistance in Medicago sativa. J. Proteom. 2017;152:109–120. doi: 10.1016/j.jprot.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 74.Zhao X., Chen Q., Wang Y., Shen Z., Shen W., Xu X. Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotoxicol. Environ. Saf. 2017;144:369–379. doi: 10.1016/j.ecoenv.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 75.Chen M., Cui W., Zhu K., Xie Y., Zhang C., Shen W. Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J. Hazard. Mater. 2014;267:40–47. doi: 10.1016/j.jhazmat.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 76.Cui W., Fang P., Zhu K., Mao Y., Gao C., Xie Y., Wang J., Shen W. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol. Environ. Saf. 2014;105:103–111. doi: 10.1016/j.ecoenv.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Hancock J.T., Hancock T.H. Hydrogen Gas, ROS Metabolism, and Cell Signaling: Are Hydrogen Spin States Important? React. Oxyg. Species. 2018;6:389–395. doi: 10.20455/ros.2018.869. [DOI] [Google Scholar]
- 78.Chen Q., Zhao X., Lei D., Hu S., Shen Z., Shen W., Xu X. Hydrogen-rich water pretreatment alters photosynthetic gas exchange, chlorophyll fluorescence, and antioxidant activities in heat-stressed cucumber leaves. Plant Growth Regul. 2017;83:1–14. doi: 10.1007/s10725-017-0284-1. [DOI] [Google Scholar]
- 79.Su N., Wu Q., Liu Y., Cai J., Shen W., Xia K., Cui J. Hydrogen-rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesis in two varieties of radish sprouts under UV-A irradiation. J. Agric. Food Chem. 2014;62:6454–6462. doi: 10.1021/jf5019593. [DOI] [PubMed] [Google Scholar]
- 80.Xie Y., Wei Z., Duan X., Chen D., Zhang Y., Cui W., Ren W., Shen W. Hydrogen-rich water-alleviated ultraviolet-B-triggered oxidative damage is partially associated with the manipulation of the metabolism of (iso)flavonoids and antioxidant defence in Medicago sativa. Funct. Plant Biol. 2015;42:1–10. doi: 10.1071/FP15204. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X., Wei J., Tian J., Li N., Jia L., Shen W., Cui J. Enhanced anthocyanin accumulation of immature radish microgreens by hydrogen-rich water under short wavelength light. Sci. Hortic. 2019;247:75–85. doi: 10.1016/j.scienta.2018.11.060. [DOI] [Google Scholar]
- 82.Jin Q., Zhu K., Cui W., Li L., Shen W. Hydrogen-modulated stomatal sensitivity to abscisic acid and drought tolerance via the regulation of apoplastic pH in Medicago sativa. J. Plant Growth Regul. 2016;35:565–573. doi: 10.1007/s00344-015-9561-2. [DOI] [Google Scholar]
- 83.Singh V.P., Singh S., Kumar J., Prasad S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate–glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015;181:20–29. doi: 10.1016/j.jplph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Tian B., Qiao Z., Zhang L., Li H., Pei Y. Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol. Biochem. 2016;109:293–299. doi: 10.1016/j.plaphy.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Mostofa M.G., Rahman A., Ansary M.M.U., Watanabe A., Fujita M., Tran L.-S.P. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015;5:14078. doi: 10.1038/srep14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng Y.-Q., Bao J., Yuan F., Liang X., Feng Z.-T., Wang B.-S. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016;79:391–399. doi: 10.1007/s10725-015-0143-x. [DOI] [Google Scholar]
- 87.Jiang J.-L., Tian Y., Li L., Yu M., Hou R.-P., Ren X.-M. H2S Alleviates Salinity Stress in Cucumber by Maintaining the Na+/K+ Balance and Regulating H2S Metabolism and Oxidative Stress Response. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma Y., Zhang W., Niu J., Ren Y., Zhang F. Hydrogen sulfide may function downstream of hydrogen peroxide in salt stress-induced stomatal closure in Vicia faba. Funct. Plant Biol. 2019;46:136–145. doi: 10.1071/FP18096. [DOI] [PubMed] [Google Scholar]
- 89.Qi Q., Guo Z., Liang Y., Li K., Xu H. Hydrogen sulfide alleviates oxidative damage under excess nitrate stress through MAPK/NO signaling in cucumber. Plant Physiol. Biochem. 2019;135:1–8. doi: 10.1016/j.plaphy.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 90.Luo Z., Li D., Du R., Mou W. Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci. Hortic. 2015;183:144–151. doi: 10.1016/j.scienta.2014.12.021. [DOI] [Google Scholar]
- 91.Li D., Limwachiranon J., Li L., Du R., Luo Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016;208:272–278. doi: 10.1016/j.foodchem.2016.03.113. [DOI] [PubMed] [Google Scholar]
- 92.Li Z.-G., Gong M., Xie H., Yang L., Li J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012;185:185–189. doi: 10.1016/j.plantsci.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H., Jiao H., Jiang C.X., Wang S.H., Wei Z.J., Luo J.P., Jones R.L. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol. Plant. 2010;32:849–857. doi: 10.1007/s11738-010-0469-y. [DOI] [Google Scholar]
- 94.Jin Z., Xue S., Luo Y., Tian B., Fang H., Li H., Pei Y. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 2013;62:41–46. doi: 10.1016/j.plaphy.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 95.Terrón-Camero L.C., Peláez-Vico M.Á., Del-Val C., Sandalio L.M., Romero-Puertas M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019;70:4477–4488. doi: 10.1093/jxb/erz184. [DOI] [PubMed] [Google Scholar]
- 96.Chen J., Xiao Q., Wang C., Wang W.-H., Wu F.-H., He B.-Y., Zhu Z., Ru Q.-M., Zhang L.-L., Zheng H.-L. Nitric oxide alleviates oxidative stress caused by salt in leaves of a mangrove species, Aegiceras corniculatum. Aquat. Bot. 2014;117:41–47. doi: 10.1016/j.aquabot.2014.04.004. [DOI] [Google Scholar]
- 97.Ahmad P., Abdel Latef A.A., Hashem A., Abd_Allah E.F., Gucel S., Tran L.-S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016;7:347. doi: 10.3389/fpls.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gupta P., Srivastava S., Seth C.S. 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil. 2017;411:483–498. doi: 10.1007/s11104-016-3043-6. [DOI] [Google Scholar]
- 99.Yadu S., Dewangan T.L., Chandrakar V., Keshavkant S. Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants. 2017;23:43–58. doi: 10.1007/s12298-016-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gadelha C.G., de Souza Miranda R., Alencar N.L.M., Costa J.H., Prisco J.T., Gomes-Filho E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant Physiol. 2017;212:69–79. doi: 10.1016/j.jplph.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Kumar R., Tasleem M., Jain M., Ahuja S., Goswami S., Bakshi S., Jambhulkar S., Singh S., Singh G., Pathak H. Nitric oxide triggered defense network in wheat: Augmenting tolerance and grain-quality related traits under heat-induced oxidative damage. Environ. Exp. Bot. 2019;158:189–204. doi: 10.1016/j.envexpbot.2018.11.016. [DOI] [Google Scholar]
- 102.Rabiei V., Kakavand F., Zaare-Nahandi F., Razavi F., Aghdam M.S. Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 2019;243:268–273. doi: 10.1016/j.scienta.2018.08.034. [DOI] [Google Scholar]
- 103.Li Z., Yong B., Cheng B., Wu X., Zhang Y., Zhang X., Peng Y. Nitric oxide, γ-aminobutyric acid, and mannose pretreatment influence metabolic profiles in white clover under water stress. J. Integr. Plant Biol. 2019 doi: 10.1111/jipb.12770. [DOI] [PubMed] [Google Scholar]
- 104.Philippe F., Verdu I., Morère-Le Paven M.-C., Limami A.M., Planchet E. Involvement of Medicago truncatula glutamate receptor-like channels in nitric oxide production under short-term water deficit stress. J. Plant Physiol. 2019;236:1–6. doi: 10.1016/j.jplph.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 105.Kolbert Z.S., Barroso J.B., Brouquisse R., Corpas F.J., Gupta K.J., Lindermayr C., Loake G.J., Palma J.M., Petřivalský M., Wendehenne D., et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide. 2019;93:53–70. doi: 10.1016/j.niox.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 106.Cui W., Fu G., Wu H., Shen W. Cadmium-induced heme oxygenase-1 gene expression is associated with the depletion of glutathione in the roots of Medicago sativa. Biometals. 2011;24:93–103. doi: 10.1007/s10534-010-9377-2. [DOI] [PubMed] [Google Scholar]
- 107.Meng D.K., Chen J., Yang Z.M. Enhancement of tolerance of Indian mustard (Brassica juncea) to mercury by carbon monoxide. J. Hazard. Mater. 2011;186:1823–1829. doi: 10.1016/j.jhazmat.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 108.Chen Q., Gong C., Ju X., Zhu Z., Shen W., Shen Z., Cui J. Hemin Through the Heme Oxygenase 1/Ferrous Iron, Carbon Monoxide System Involved in Zinc Tolerance in Oryza Sativa L. J. Plant Growth Regul. 2018;37:947–957. doi: 10.1007/s00344-018-9793-z. [DOI] [Google Scholar]
- 109.Huang B.K., Xu S., Xuan W., Li M., Cao Z.Y., Liu K.L., Ling T.F., Shen W.B. Carbon monoxide alleviates salt-induced oxidative damage in wheat seedling leaves. J. Integr. Plant Biol. 2006;48:249–254. doi: 10.1111/j.1744-7909.2006.00220.x. [DOI] [Google Scholar]
- 110.Xu S., Sa Z.S., Cao Z.Y., Xuan W., Huang B.K., Ling T.F., Hu Q.Y., Shen W.B. Carbon monoxide alleviates wheat seed germination inhibition and counteracts lipid peroxidation mediated by salinity. J. Integr. Plant Biol. 2006;48:1168–1176. doi: 10.1111/j.1744-7909.2006.00337.x. [DOI] [Google Scholar]
- 111.Liu K., Xu S., Xuan W., Ling T., Cao Z., Huang B., Sun Y., Fang L., Liu Z., Zhao N. Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci. 2007;172:544–555. doi: 10.1016/j.plantsci.2006.11.007. [DOI] [Google Scholar]
- 112.Ling T., Zhang B., Cui W., Wu M., Lin J., Zhou W., Huang J., Shen W. Carbon monoxide mitigates salt-induced inhibition of root growth and suppresses programmed cell death in wheat primary roots by inhibiting superoxide anion overproduction. Plant Sci. 2009;177:331–340. doi: 10.1016/j.plantsci.2009.06.004. [DOI] [Google Scholar]
- 113.Zhang C., Li Y., Yuan F., Hu S., He P. Effects of hematin and carbon monoxide on the salinity stress responses of Cassia obtusifolia L. seeds and seedlings. Plant Soil. 2012;359:85–105. doi: 10.1007/s11104-012-1194-7. [DOI] [Google Scholar]
- 114.Liu Y., Xu S., Ling T., Xu L., Shen W. Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J. Plant Physiol. 2010;167:1371–1379. doi: 10.1016/j.jplph.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 115.Wu H., Zheng Y., Liu J., Zhang H., Chen H. Heme oxygenase-1 delays gibberellin-induced programmed cell death of rice aleurone layers subjected to drought stress by interacting with nitric oxide. Front. Plant Sci. 2016;6:1267. doi: 10.3389/fpls.2015.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su J., Zhang Y., Nie Y., Cheng D., Wang R., Hu H., Chen J., Zhang J., Du Y., Shen W. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ. Exp. Bot. 2018;147:249–260. doi: 10.1016/j.envexpbot.2017.12.022. [DOI] [Google Scholar]
- 117.Zhu Y., Liao W., Niu L., Wang M., Ma Z. Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol. 2016;16:146. doi: 10.1186/s12870-016-0834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corpas F.J. Nitric Oxide and Hydrogen Sulfide in Higher Plants under Physiological and Stress Conditions. Multidisciplinary Digital Publishing Institute; Basel, Switzerland: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie Y., Ling T., Han Y., Liu K., Zheng Q., Huang L., Yuan X., He Z., Hu B., Fang L. Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ. 2008;31:1864–1881. doi: 10.1111/j.1365-3040.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 120.Santa-Cruz D.M., Pacienza N.A., Polizio A.H., Balestrasse K.B., Tomaro M.L., Yannarelli G.G. Nitric oxide synthase-like dependent NO production enhances heme oxygenase up-regulation in ultraviolet-B-irradiated soybean plants. Phytochemistry. 2010;71:1700–1707. doi: 10.1016/j.phytochem.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 121.Cui W., Qi F., Zhang Y., Cao H., Zhang J., Wang R., Shen W. Methane-rich water induces cucumber adventitious rooting through heme oxygenase1/carbon monoxide and Ca2+ pathways. Plant Cell Rep. 2015;34:435–445. doi: 10.1007/s00299-014-1723-3. [DOI] [PubMed] [Google Scholar]
- 122.Astier J., Lindermayr C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012;13:15193–15208. doi: 10.3390/ijms131115193. [DOI] [PMC free article] [PubMed] [Google Scholar]