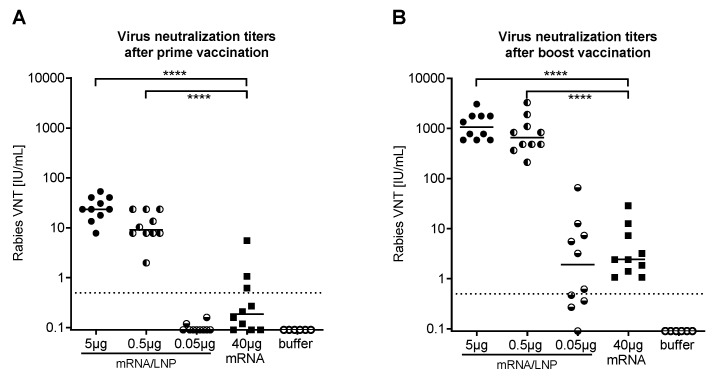
Figure 4.
Serum neutralizing antibody responses to mRNA-LNP formulations in mice (adapted from reference [41]). (A) shows rabies virus neutralizing antibody titers (VNT) measured in serum of Balb/c mice three weeks after the second of two intradermal vaccinations given 3 weeks apart; vaccinations consisted of 0.05, 0.5, or 5 μg mRNA-LNP or 40 μg nonformulated mRNA or buffer. (B) shows VNT titers measured in serum of Balb/c mice two weeks after the mice received a booster dose of the same vaccine five months after the primary doses. Groups of ten mice were in each case. Dashed line shows WHO protective value (0.5 IU/mL), **** = p < 0.0001; Lipid nanoparticle (LNP)-formulated mRNA was generated using LNPs provided by Acuitas Therapeutics (Canada).