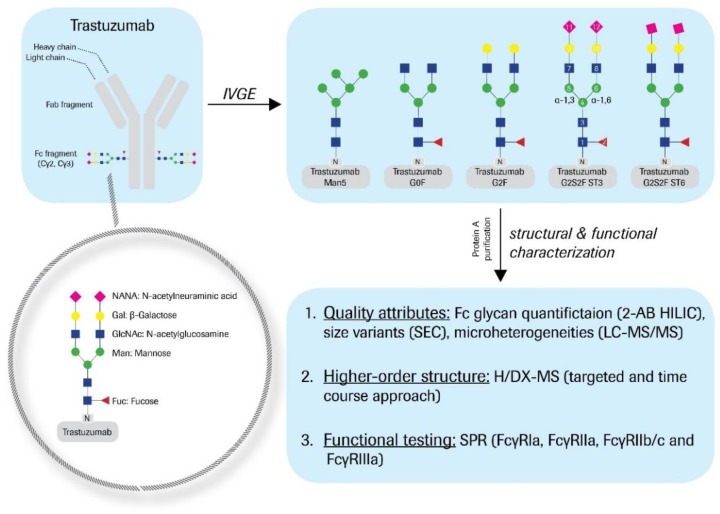
Figure 1.
Study design. Trastuzumab drug substance (DS) was in vitro glyco-engineered (IVGE) with individual enzymatic post-process treatment, as described in the methods section. The trastuzumab Fc glyco-variants obtained (as determined by 2-AB HILIC; see Table 1 for details): G0F (81%), G2F (83%), G2S2F ST3 (60%), and G2S2F ST6 (43%). An additional trastuzumab Man5 variant (88%) was prepared by kifunensine treatment during the fermentation processing. All glyco-variants were protein A purified after IVGE treatment and further structurally and functionally characterized with 2-AB HILIC, SEC, LC-MS/MS (quality attributes), HDX-MS (higher-order structure), and SPR (functional testing).