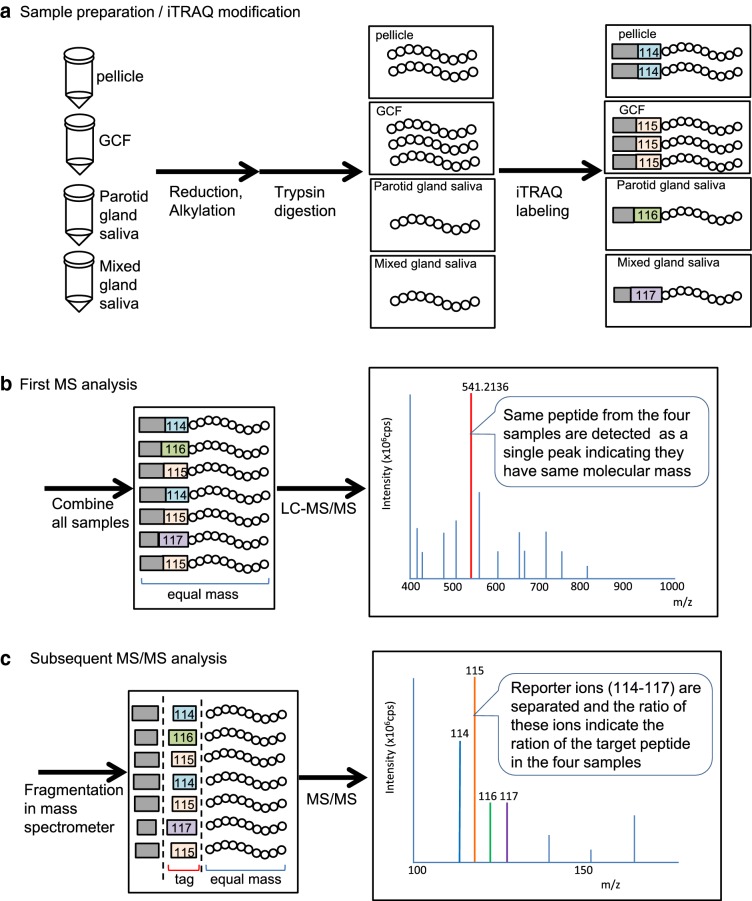
Fig. 2.
Schematic illustration of iTRAQ method of proteomic relative quantitation. a Pretreatment of the samples before LC–MS/MS analysis. A set of four samples from the same subject (5 μg each) was treated with dithiothreitol and iodoacetamide to cleave disulfide bonds followed by tryptic digestion. Then, amino termini of the peptides were labeled with either one of iTRAQ reagents 114, 115, 116 or 117. The iTARQ reagents have a same molecular mass but they produce isobaric tag moieties with different masses when cleaved during MS/MS analysis. b The iTRAQ-labeled four samples were mixed, and then the mixture was analyzed by LC–MS/MS. In the first MS analysis, the labeled peptides with the same molecular mass are detected as a single peptide. c The instrument subsequently breaks the peptides with high voltage and the fragments were analyzed. In this step, isobaric tags generated were detected separately. The ratio of the tags represents the ratio of the peptides in the four samples. In the same time, fragments of the peptides provide structural information about amino acid sequence