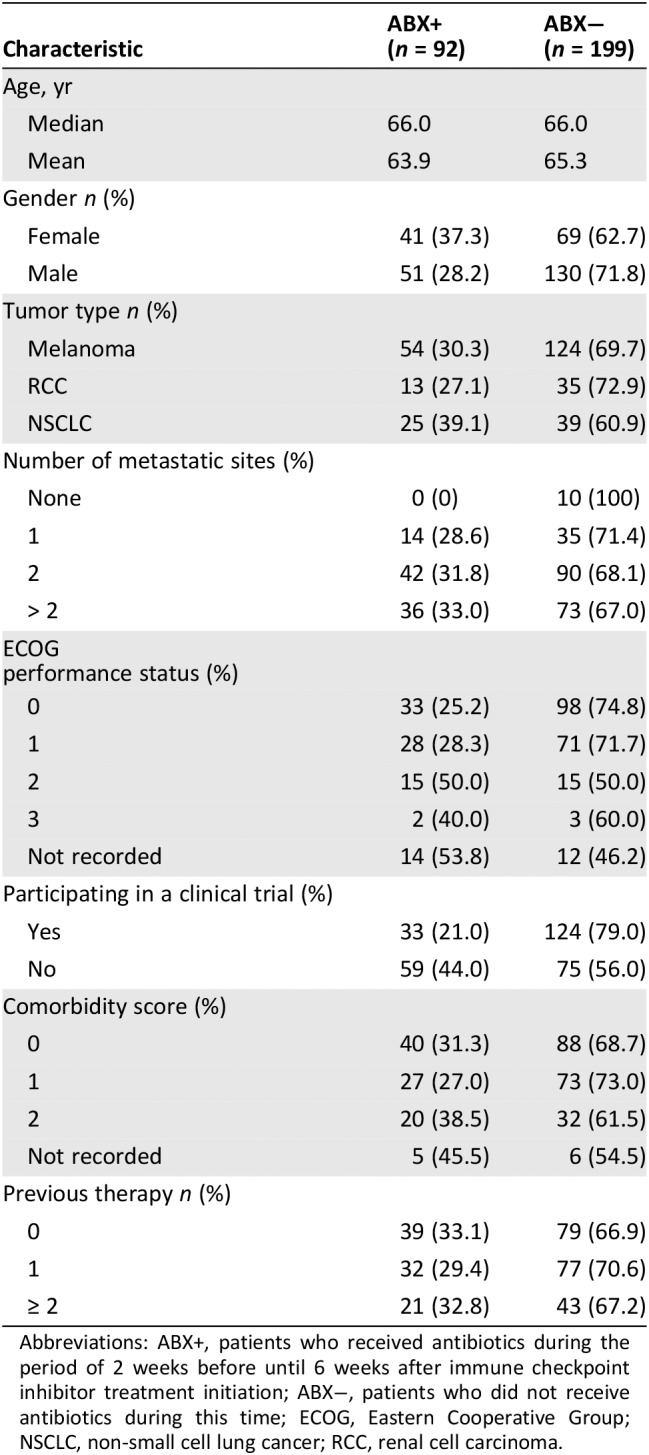
Table 1.
Characteristics of patients evaluated in the study
| Characteristic | ABX+ (n = 92) | ABX− (n = 199) |
|---|---|---|
| Age, yr | ||
| Median | 66.0 | 66.0 |
| Mean | 63.9 | 65.3 |
| Gender n (%) | ||
| Female | 41 (37.3) | 69 (62.7) |
| Male | 51 (28.2) | 130 (71.8) |
| Tumor type n (%) | ||
| Melanoma | 54 (30.3) | 124 (69.7) |
| RCC | 13 (27.1) | 35 (72.9) |
| NSCLC | 25 (39.1) | 39 (60.9) |
| Number of metastatic sites (%) | ||
| None | 0 (0) | 10 (100) |
| 1 | 14 (28.6) | 35 (71.4) |
| 2 | 42 (31.8) | 90 (68.1) |
| > 2 | 36 (33.0) | 73 (67.0) |
|
ECOG performance status (%) |
||
| 0 | 33 (25.2) | 98 (74.8) |
| 1 | 28 (28.3) | 71 (71.7) |
| 2 | 15 (50.0) | 15 (50.0) |
| 3 | 2 (40.0) | 3 (60.0) |
| Not recorded | 14 (53.8) | 12 (46.2) |
| Participating in a clinical trial (%) | ||
| Yes | 33 (21.0) | 124 (79.0) |
| No | 59 (44.0) | 75 (56.0) |
| Comorbidity score (%) | ||
| 0 | 40 (31.3) | 88 (68.7) |
| 1 | 27 (27.0) | 73 (73.0) |
| 2 | 20 (38.5) | 32 (61.5) |
| Not recorded | 5 (45.5) | 6 (54.5) |
| Previous therapy n (%) | ||
| 0 | 39 (33.1) | 79 (66.9) |
| 1 | 32 (29.4) | 77 (70.6) |
| ≥ 2 | 21 (32.8) | 43 (67.2) |
Abbreviations: ABX+, patients who received antibiotics during the period of 2 weeks before until 6 weeks after immune checkpoint inhibitor treatment initiation; ABX−, patients who did not receive antibiotics during this time; ECOG, Eastern Cooperative Group; NSCLC, non‐small cell lung cancer; RCC, renal cell carcinoma.
