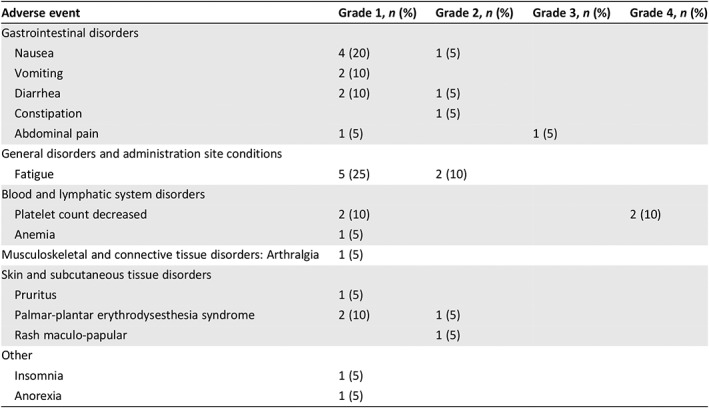
Table 2.
Treatment‐related adverse events (per Common Terminology Criteria for Adverse Events, version 4.03)
| Adverse event | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| Nausea | 4 (20) | 1 (5) | ||
| Vomiting | 2 (10) | |||
| Diarrhea | 2 (10) | 1 (5) | ||
| Constipation | 1 (5) | |||
| Abdominal pain | 1 (5) | 1 (5) | ||
| General disorders and administration site conditions | ||||
| Fatigue | 5 (25) | 2 (10) | ||
| Blood and lymphatic system disorders | ||||
| Platelet count decreased | 2 (10) | 2 (10) | ||
| Anemia | 1 (5) | |||
| Musculoskeletal and connective tissue disorders: Arthralgia | 1 (5) | |||
| Skin and subcutaneous tissue disorders | ||||
| Pruritus | 1 (5) | |||
| Palmar‐plantar erythrodysesthesia syndrome | 2 (10) | 1 (5) | ||
| Rash maculo‐papular | 1 (5) | |||
| Other | ||||
| Insomnia | 1 (5) | |||
| Anorexia | 1 (5) |