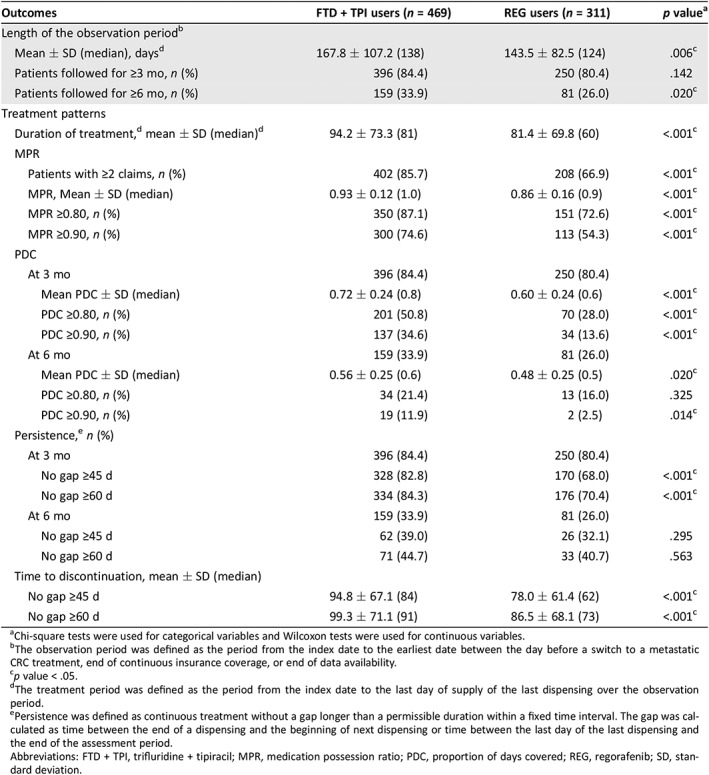
Table 2.
Treatment patterns, adherence, and persistence postindex date
| Outcomes | FTD + TPI users (n = 469) | REG users (n = 311) | p valuea |
|---|---|---|---|
| Length of the observation periodb | |||
| Mean ± SD (median), daysd | 167.8 ± 107.2 (138) | 143.5 ± 82.5 (124) | .006c |
| Patients followed for ≥3 mo, n (%) | 396 (84.4) | 250 (80.4) | .142 |
| Patients followed for ≥6 mo, n (%) | 159 (33.9) | 81 (26.0) | .020c |
| Treatment patterns | |||
| Duration of treatment,d mean ± SD (median)d | 94.2 ± 73.3 (81) | 81.4 ± 69.8 (60) | <.001c |
| MPR | |||
| Patients with ≥2 claims, n (%) | 402 (85.7) | 208 (66.9) | <.001c |
| MPR, Mean ± SD (median) | 0.93 ± 0.12 (1.0) | 0.86 ± 0.16 (0.9) | <.001c |
| MPR ≥0.80, n (%) | 350 (87.1) | 151 (72.6) | <.001c |
| MPR ≥0.90, n (%) | 300 (74.6) | 113 (54.3) | <.001c |
| PDC | |||
| At 3 mo | 396 (84.4) | 250 (80.4) | |
| Mean PDC ± SD (median) | 0.72 ± 0.24 (0.8) | 0.60 ± 0.24 (0.6) | <.001c |
| PDC ≥0.80, n (%) | 201 (50.8) | 70 (28.0) | <.001c |
| PDC ≥0.90, n (%) | 137 (34.6) | 34 (13.6) | <.001c |
| At 6 mo | 159 (33.9) | 81 (26.0) | |
| Mean PDC ± SD (median) | 0.56 ± 0.25 (0.6) | 0.48 ± 0.25 (0.5) | .020c |
| PDC ≥0.80, n (%) | 34 (21.4) | 13 (16.0) | .325 |
| PDC ≥0.90, n (%) | 19 (11.9) | 2 (2.5) | .014c |
| Persistence,e n (%) | |||
| At 3 mo | 396 (84.4) | 250 (80.4) | |
| No gap ≥45 d | 328 (82.8) | 170 (68.0) | <.001c |
| No gap ≥60 d | 334 (84.3) | 176 (70.4) | <.001c |
| At 6 mo | 159 (33.9) | 81 (26.0) | |
| No gap ≥45 d | 62 (39.0) | 26 (32.1) | .295 |
| No gap ≥60 d | 71 (44.7) | 33 (40.7) | .563 |
| Time to discontinuation, mean ± SD (median) | |||
| No gap ≥45 d | 94.8 ± 67.1 (84) | 78.0 ± 61.4 (62) | <.001c |
| No gap ≥60 d | 99.3 ± 71.1 (91) | 86.5 ± 68.1 (73) | <.001c |
Chi‐square tests were used for categorical variables and Wilcoxon tests were used for continuous variables.
The observation period was defined as the period from the index date to the earliest date between the day before a switch to a metastatic CRC treatment, end of continuous insurance coverage, or end of data availability.
p value < .05.
The treatment period was defined as the period from the index date to the last day of supply of the last dispensing over the observation period.
Persistence was defined as continuous treatment without a gap longer than a permissible duration within a fixed time interval. The gap was calculated as time between the end of a dispensing and the beginning of next dispensing or time between the last day of the last dispensing and the end of the assessment period.
Abbreviations: FTD + TPI, trifluridine + tipiracil; MPR, medication possession ratio; PDC, proportion of days covered; REG, regorafenib; SD, standard deviation.
