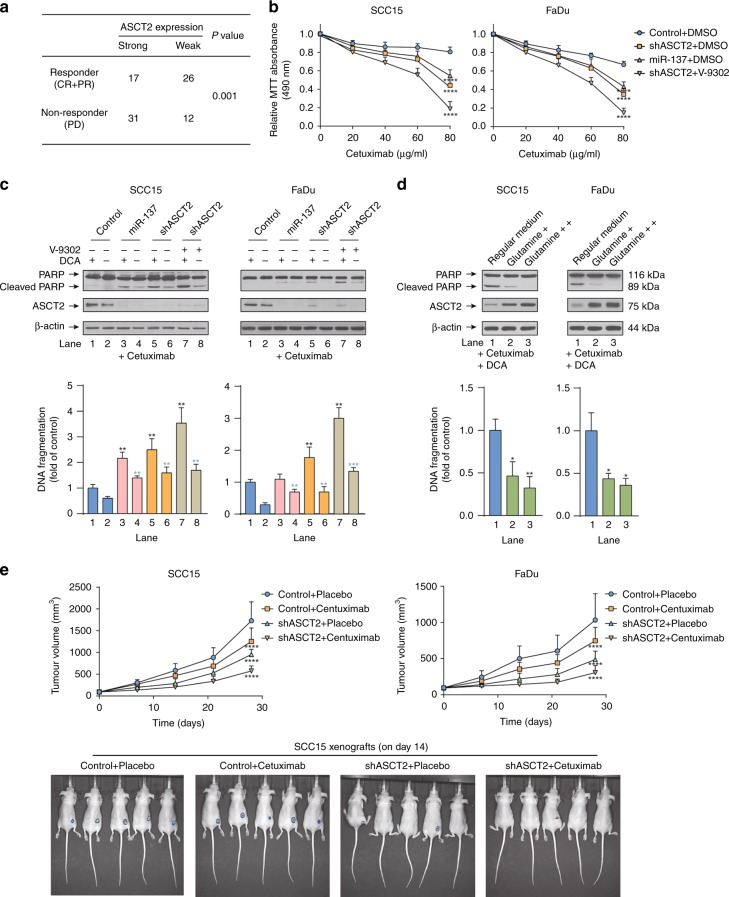
Fig. 6.
Targeting ASCT2 improved the response of HNSCC to cetuximab. a HNSCC patients treated with cetuximab were divided into two groups according to their ASCT2 expression as measured by immunohistochemistry. The chi-square test was used to evaluate the relationship between response to cetuximab and ASCT2 expression. The responders included complete responders (CRs) and partial responders (PRs), while non-responders were patients with progressive disease (PD). b The MTT assay showed that the inhibition of proliferation by cetuximab was enhanced by transfection with the two individual ASCT2 shRNAs or miR-137 or the combination of shASCT2 with V-9302 treatment (25 µM, 48 h). c SCC15 and FaDu cells infected with a lentiviral vector expressing ASCT2 shRNA or a control vector or treated with either DMSO or V-9302 (25 µM, 48 h) were treated with 20 nM cetuximab with or without 10 mM DCA. Cell lysates were subjected to western blotting for the detection of PARP cleavage. The results were verified by apoptosis ELISA. d The cells were treated with cetuximab plus DCA for 24 h in regular medium supplemented with additional glutamine. The results were verified by apoptosis ELISA. e SCC15 or FaDu cells were infected with a pLEX-based recombinant lentivirus containing firefly luciferase cDNA with and without targeted ASCT2 interference and then implanted subcutaneously into the right flanks of nude mice (6 × 1006 cells/mouse in 100 ml of serum-free medium). Treatment with vehicle or cetuximab (250 mg/mouse twice a week) started at 9 days after injection for FaDu cells and at 6 days after injection for SCC15 cells. Images of the mice on day 14 (cetuximab or vehicle initiation as day 1) are shown. ‘*’, ‘**’, ‘***’ and ‘****’ represent ‘p < 0.05′, ‘p < 0.01′, ‘p < 0.001′ and ‘p < 0.0001′, respectively (‘*’ was coloured black with reference to the line 1, ‘*’ was coloured blue with reference to the line 2)