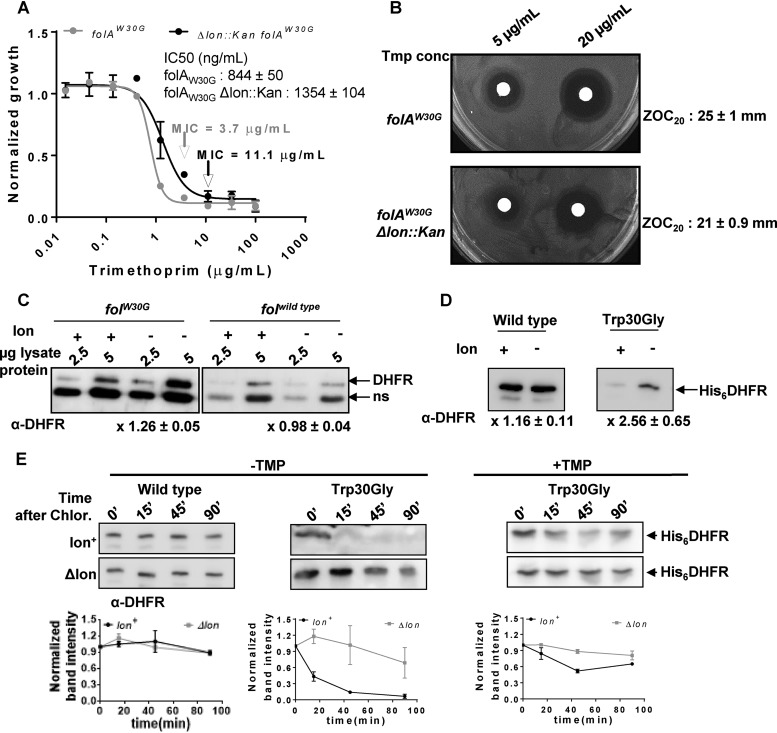
FIG 4.
Lon deficiency potentiates the trimethoprim resistance level of a spontaneous drug-resistant isolate by increasing the in vivo stability of mutant DHFR. (A) Dose-response curves for a trimethoprim-resistant isolate of E. coli harboring the folAW30G allele and its Δlon::kan derivative. Peak optical density after 15 to 18 h of growth at each trimethoprim concentration was normalized to growth in drug-free medium (normalized growth). Means ± SD of results from 3 independent experiments are plotted. The drug MIC values for each of the strains are indicated in the graph with an arrow. IC50 values (means ± SEM) estimated from the data are provided as an inset. (B) Trimethoprim resistance of E. coli folAW30G and its Δlon::kan derivative assessed by a disc diffusion assay at the indicated concentrations of trimethoprim. Diameters of zone of clearance were estimated at 20 μg/ml of the antibiotic (ZOC20). Means ± SD of the diameters of the ZOC from 3 independent experiments are shown. (C) Levels of endogenous DHFR in lysates of drug-sensitive (folAwild type) or drug-resistant (folAW30G) E. coli strains and their Δlon::kan derivatives assayed by immunoblotting. DHFR polyclonal antibody (α-DHFR) was used as the primary antibody. Specific (DHFR) and nonspecific (ns) immune-reactive bands are indicated. Two different amounts of lysate protein (2.5 μg and 5 μg) were used for each strain to ensure that the Western blot was not saturated. Fold expression of DHFR (Δlon/wild type) was calculated using densitometric analysis of band intensities. Means ± SD of results from 3 independent experiments are indicated below the image. (D) Levels of plasmid-encoded wild type or Trp30Gly mutant DHFR (His6DHFR) in Lon-deficient Lon-expressing E. coli assayed by immunoblotting using DHFR polyclonal antibody (α-DHFR). Fold expression of DHFR (Δlon/wild type) was calculated using densitometric analysis of band intensities. Means ± SD of results from 3 independent experiments are indicated below the image. (E) In vivo stabilities of plasmid-encoded wild-type or Trp30Gly mutant DHFR (His6DHFR) in Lon-deficient or Lon-expressing E. coli assayed using a chloramphenicol (Chlor.) chase shutoff assay. After inhibition of protein synthesis by addition of chloramphenicol, levels of DHFR were assessed using immunoblotting with DHFR-specific polyclonal antibody (α-DHFR) at the indicated times. For Trp30Gly DHFR, the assay was performed in the presence or absence of trimethoprim in the growth medium as indicated. Representative immunoblot data from experiments performed at least thrice are shown. Below each blot, quantitation of band intensities normalized to the intensity measured at 0 min is plotted as means ± SD of results from 3 independent experiments.