Figure 1.
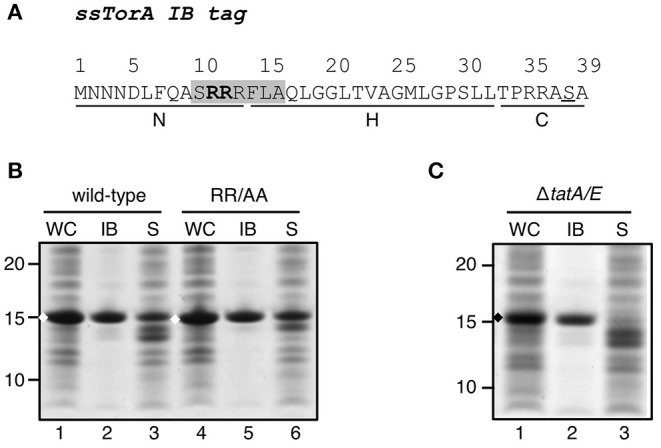
Influence of a functional Tat-system on IB-formation by ssTorA. (A). Amino acid sequence of the ssTorA IB formation tag. The consensus sequence containing a twin-arginine pair (bold) that is conserved among Tat-signal sequences is shaded (Berks, 1996). The basic N-region, hydrophobic H-region and polar C-region, which are common for signal sequences (von Heijne, 1985), are indicated below the amino acid sequence (Cristobal et al., 1999). A serine residue that replaces a native threonine in ssTorA (Jong et al., 2017) is underlined. (B) IB sedimentation assay on E. coli TOP10F′ cells expressing a TrxA fusion carrying wild-type ssTorA as displayed under A or derivative ssTorA(RR/AA). Cells were lysed and subjected to differential centrifugation to separate the inclusion bodies (IB)-containing insoluble fraction from the soluble cell fraction (S). Samples of both fractions and corresponding whole cell (WC) samples were analyzed by SDS-PAGE and Coomassie staining. All samples were derived from the same amount of cell material. (C) IB-sedimentation assay on MC4100ΔtatA/E cells expressing ssTorA/TrxA as described under B. Bands representing ssTorA/TrxA fusions of interest are indicated (♦). Molecular mass (kDa) markers are indicated at the left side of the panels.
