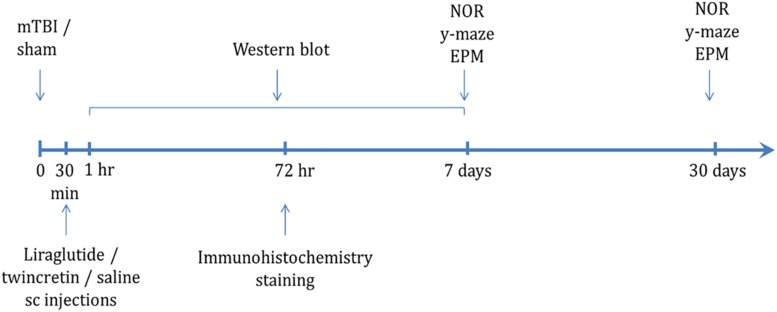
FIGURE 1.
Scheme of study design time line. Animals were exposed to sham/mTBI and 30 min later were given liraglutide (247.6 μg/kg), twincretin (50 μg/kg), or saline once daily via s.c. injections for 7 days {the human dose of liraglutide and twincretin is 1.8 mg s.c. daily that, for a 88.8 kg human [the mean weight of a male American (Yuan et al., 2017)], following normalization to body surface area across species in line with U.S. Department of Health and Human Services Food and Drug Administration guidelines (Han et al., 2016), translates to a mouse dose of approximately 247.6 μg/kg}. Behavioral tests to assess behavior and cognitive abilities were carried out 7 and 30 days following mTBI in separate cohorts of animals. Immunohistochemical staining to evaluate neurodegeneration and neuroinflammation was performed 72 h following mTBI challenge. Western blot analysis to assess changes in neuroprotective protein levels following mTBI and drug treatments was performed starting from 1 h to 1 week post mTBI. Each experimental time point was performed using a different group of mice (NOR: novel object recognition paradigm; EPM: elevated plus maze paradigm).