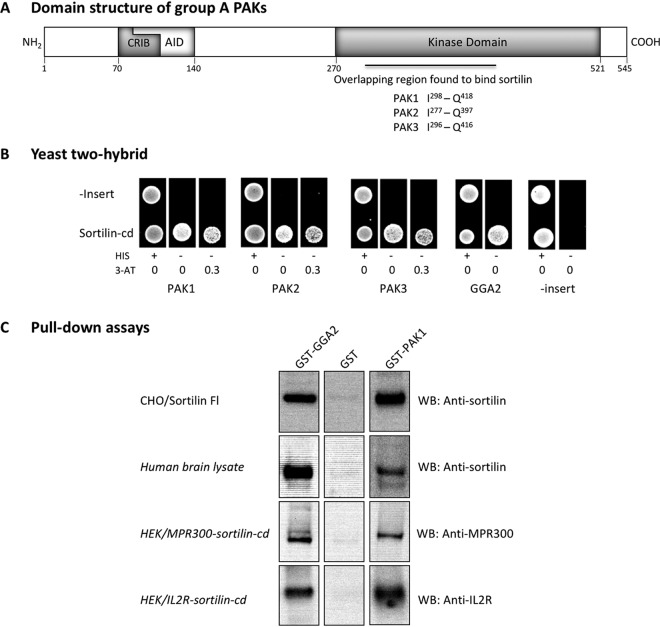
FIG 1.
Interaction of the kinase domain of PAK1-3 with the cytoplasmic domain of sortilin. (A) Domain structure of group A PAKs. The amino acid numbering is according to human PAK1 (GenBank accession number NP_002567.3). The PAK1-3 kinases contain an N-terminal GTPase-binding domain with a Cdc42/Rac-interactive binding (CRIB) motif partly overlapped by an autoinhibitory domain (AID) and a separate kinase domain in the C terminus. The region in the kinase domain found to bind sortilin for all three kinases is marked. (B) Confirmation of interaction between sortilin and PAK kinases by yeast two-hybrid analysis. The cytoplasmic domain of human sortilin (sortilin-cd) was cloned into the Hybrigenics bait vector and tested against prey plasmids encoding human PAK1-3 or GGA2, including PAK1-T271-H545, PAK2-K146-Q397, PAK3-I317-R565, and GGA2-S37-N202. Growing colonies indicate interactions between expressed proteins. His, histidine; 3-AT, 3-amino-1,2,4-triazole. (C) Pulldown assays with GST-tagged PAK1 kinase. Full-length sortilin was pulled down from lysates of CHO/Sortilin cells and human brain and detected by Western blotting using antibodies against the sortilin ectodomain. Chimeric receptors combining the sortilin-cd with the ectodomain of either the mannose-6-phosphate receptor (MPR300–sortilin-cd) or the interleukin-2 receptor (IL-2R–sortilin-cd) were precipitated from HEK cell transfectants and detected using anti-MPR300 and anti-IL-2R antibodies, respectively. GGA2 and GST served as positive and negative controls.