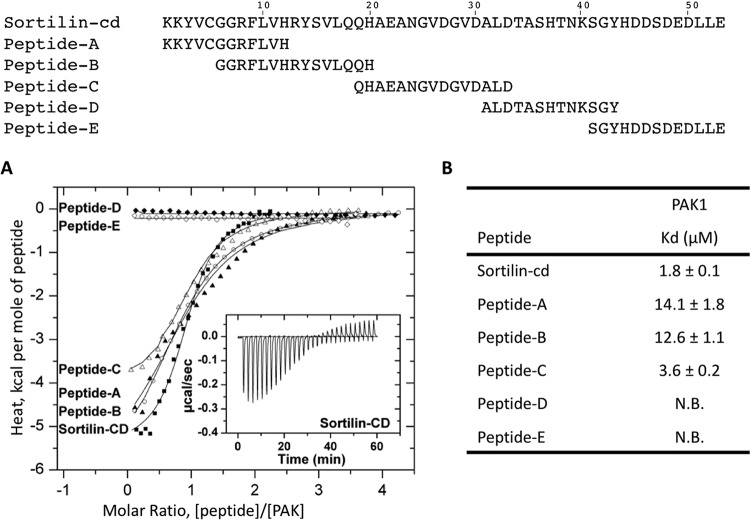
FIG 2.
Isothermal titration calorimetry analysis of PAK1 kinase binding to the cytoplasmic domain of sortilin (sortilin-cd) or peptides thereof. (A) Graphs showing integrated heat pulses, normalized per mole of peptide injected as a function of the molar ratio (peptide/PAK1 concentration). These binding curves were fitted to the one set of sites model. The calorimetric cell contained PAK1 (25 μM), and the injection syringe contained either 100 μM sortilin-cd or peptides A to E. Squares, sortilin-cd; filled triangles, peptide A; circles, peptide B; empty triangles, peptide C; filled diamonds, peptide D; empty diamonds, peptide E. The inset graph shows raw data for the heat pulses resulting from titration of 100 μM sortilin-cd in 25 μM PAK1 in 28 injections. The trace is shown after subtraction of the heat of dilution of the peptide in buffer. (B) Dissociation constants (Kd) for the different peptide-PAK1 interactions expressed as means ± standard deviations. N.B., no binding.