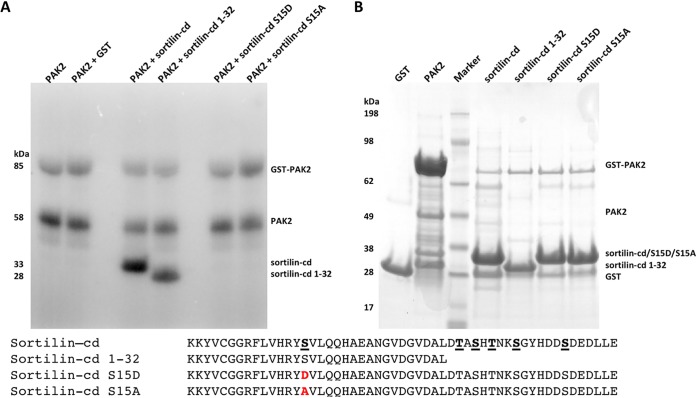
FIG 5.
In vitro phosphorylation of sortilin by group A PAK kinases. (A) In vitro phosphorylation of sortilin with a constitutively active PAK2 kinase. GST-tagged constructs of the sortilin cytoplasmic domain were expressed and purified, including the sortilin-cd, a cytoplasmic domain deletion mutant (sortilin-cd 1–32) containing only a single potential Ser phosphorylation site, and two single point mutants of Ser15 in the sortilin-cd, sortilin-cd S15A and sortilin-cd S15D. The in vitro phosphorylation assay was performed using 32P-labeled ATP, full-length GST-PAK2 T402E, and the purified fusion protein or GST as a negative control. The phosphorylation reaction was stopped after 30 min and analyzed by SDS-PAGE and subsequently autoradiography. (B) Evaluation of all isolated GST-tagged fusion proteins used for the in vitro phosphorylation assay analyzed by SDS-PAGE and Coomassie brilliant blue staining. Amino acid sequences of the sortilin cytoplasmic domain are shown, with the potential Ser and Thr phosphorylation sites underlined. Amino acid residues mutated in sortilin-cd S15D/A are marked in red.