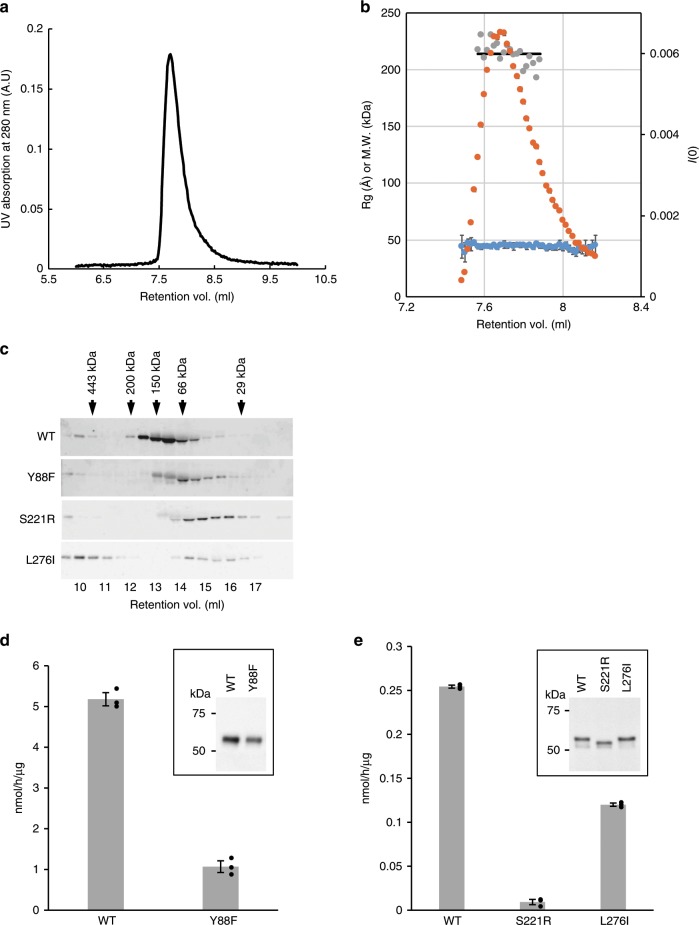
Fig. 2. Oligomerization analysis.
Oligomerization states in solution and enzymatic activities of wild-type and disease-related mutants of sFKRP were studied. SEC-SAXS profiles of wild-type sFKRP are shown in (a) (UV absorption) and (b). In (b), the SAXS intensity I(0) (orange), radii of gyration (Rg) (blue), and estimated molecular weight from Porod volume (gray) are plotted. a Black line indicates the average of estimated molecular weight from the Porod volumes. c Expressed sFKRP proteins were analyzed by usual SEC and detected by SDS-PAGE (5–20% acrylamide; ATTO) followed by Western blotting. Arrows indicate fractions at which protein markers were eluted. d, e Enzymatic activities of sFKRP with CDP-Rbo and the RboP-(phospho-)core M3 peptide. Insets: immunoblot analyses of sFKRP proteins to normalize input sFKRP. d sFKRP (WT and Y88F) immunoprecipitated from the culture supernatant were used as the enzyme sources. e Cell lysates expressing sFKRP (WT, S221R, and L276I) were used as the enzyme sources. Average values ± SE of three independent experiments are shown. Each dot represents one data point. Source data are provided as a Source Data file.