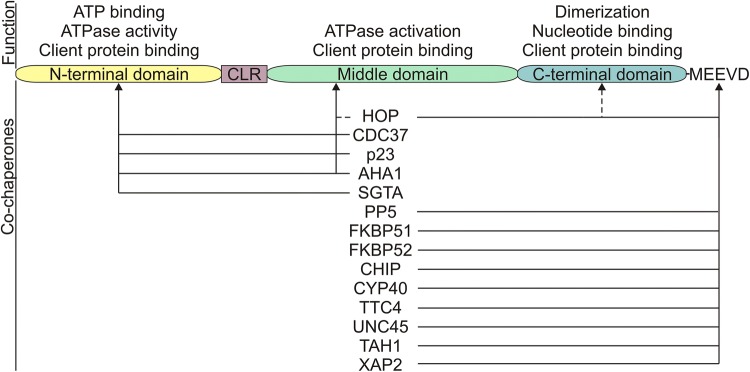
Fig. 1.
Schematic representation of HSP90 protein domain structure. Functions of each domain and HSP90-interacting co-chaperones with their binding sites are shown. Dashed lines represent alternative binding sites. AHA1: activator of HSP90 ATPase protein 1; ATP: adenosine triphosphate; CDC37: cell division cycle 37; CHIP: carboxyl terminus of HSP70-interacting protein; CLR: charged linker region; CYP40: cyclophilin 40; FKBP51: FK506-binding protein 5; FKBP52: FK506-binding protein 4; HOP: homeodomain-only protein; PP5: protein phosphatase 5; SGTA: small glutamine rich tetratricopeptide repeat-containing alpha; TAH1: telomere-associated homeobox-containing protein 1; TTC4: tetratricopeptide repeat domain 4; UNC45: smooth muscle cell-associated protein 1; XAP2: HBV X-associated protein 2