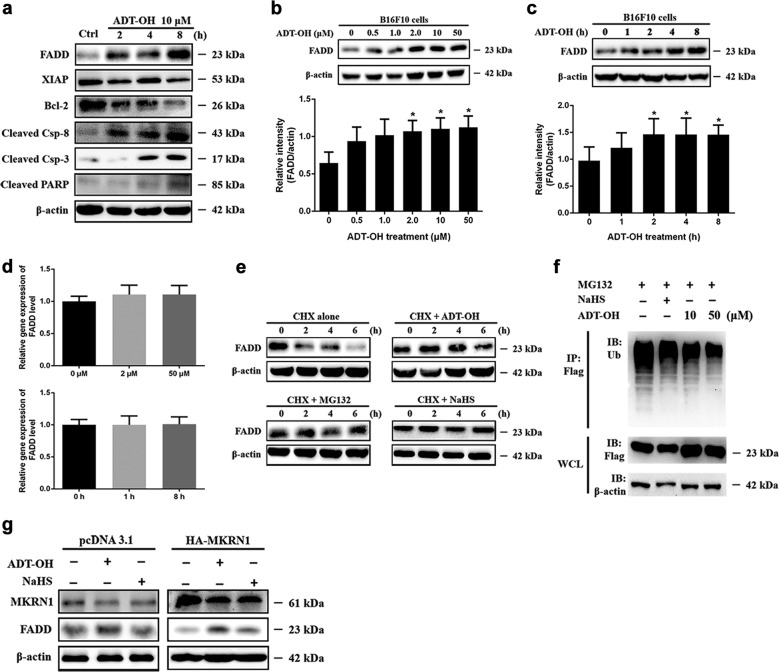
Fig. 2. ADT-OH suppresses the ubiquitin degradation of FADD through downregulating MKRN1 expression to trigger extrinsic apoptosis.
a Western blot analysis of cleaved-caspase 3, cleaved-caspase 8, cleaved-PARP, Bcl-2, XIAP and FADD in B16F10 cells treated with ADT-OH (10 μM) at different time point as indicated. b, c Western blot analysis of FADD expression in B16F10 cells after ADT-OH treated in dose- (b 2 h) and time- (c 10 μM) dependent manner. d The mRNA levels of FADD in B16F10 cells after ADT-OH treated at different concentrations and time points as indicated. e B16F10 cells were treated with 40 μg/ml of CHX for the indicated time to determine protein stability of FADD in the absence or presence of MG132 (10 μM), NaHS (2 mM), and ADT-OH (10 μM). Cells were lysed and analysed by WB using anti-FADD and anti-actin antibodies. f B16F10 cells were transfected with the FADD-Flag and HA-Ub plasmid, treated with MG132 (10 μM) for 6 h, NaHS 2 mM or ADT-OH in the the indicated concentrations for 6 h, then lysed in 1% SDS buffer. Flag-FADD was purified by immunoprecipitation using an anti-FLAG antibody, followed by WB analysis using an anti-HA antibody. g The effect of ADT-OH on MKRN1 and FADD protein levels by WB analysis. B16F10 cells were transfected with FADD in the absence or presence of MKRN1, NaHS and ADT-OH for 24 h and then treated with CHX (40 μg/ml) for 6 h. *P < 0.05, **P < 0.01. Data are expressed as mean ± SD of three independent experiments.