Figure 6.
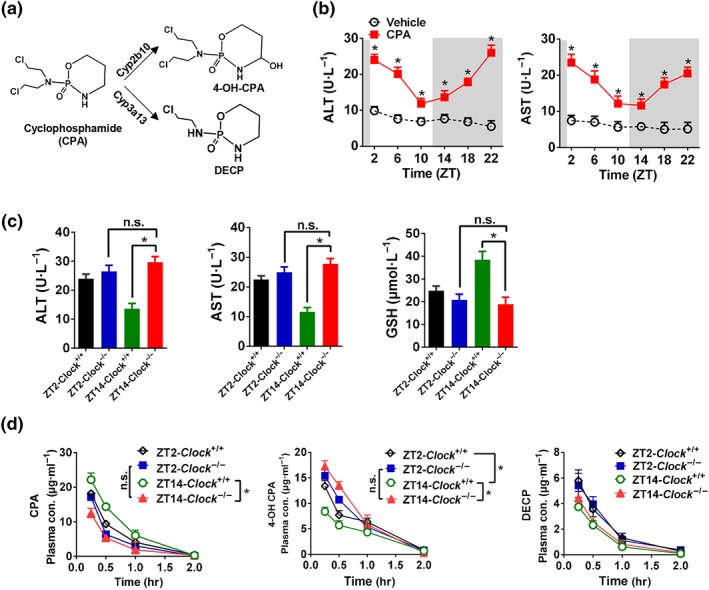
Clock ablation exacerbates cyclophosphamide toxicity and modulates its diurnal rhythm. (a) Major metabolic pathways for cyclophosphamide (CPA). (b) Cyclophosphamide hepatotoxicity shows a diurnal rhythm. Plasma ALT and AST levels in Clock +/+ mice were tested 4 hr after vehicle or cyclophosphamide administration (i.p., 300 mg·kg−1, n = 5) at six time points (ZT2, 6, 10, 14, 18, and 22). significantly different from vehicle; two‐way ANOVA and Bonferroni post hoc test (c) Plasma ALT, AST, and GSH levels in Clock +/+ and Clock −/− mice 4 hr after vehicle or cyclophosphamide administration (i.p., 300 mg·kg−1, n = 5) at ZT2 and ZT14. * P < .05, significantly different as indicated; two‐way ANOVA and Bonferroni post hoc test. (d) Plasma concentrations of cyclophosphamide, 4‐OH‐CPA, and DECP in Clock +/+ and Clock −/− mice at 0.25, 0.5, 1, and 2 hr after cyclophosphamide treatment (i.p., 100 mg·kg−1, n = 5) at ZT2 and ZT14. * P < .05, significantly different as indicated; two‐way ANOVA and Bonferroni post hoc test. Data are presented as mean ± SD. n.s., not significant
