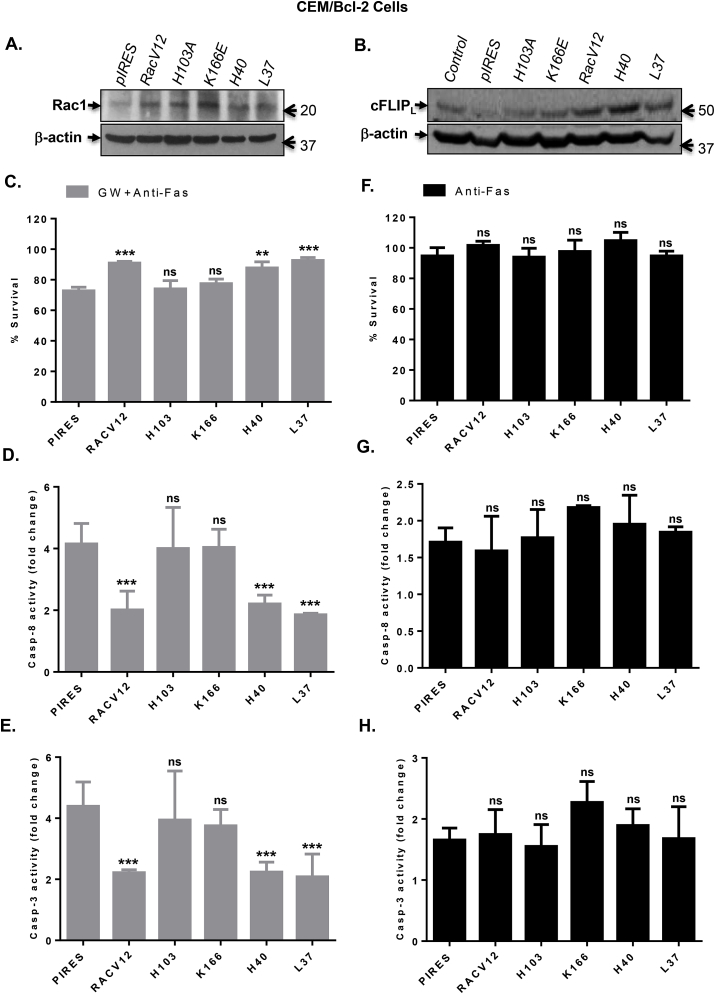
Fig. 4.
Rac1 functional mutants that retain O2•-producing activity inhibit GW-induced sensitization to anti-Fas by upregulating cFLIP. (A) Transient transfection of CEM/Bcl-2 cells with the empty pIRES vector or various Rac1 mutants (RacV12, H103A, K166E, H40, and L37) was performed as described in Material and Methods. After 48 hrs of transfection, the expression of transiently expressed proteins was detected by Western blot using anti-Rac1 with β-actin expression as the loading control. (B) cFLIP expression in lysates from CEM/Bcl-2 cells transfected with the various Rac1 mutants was assessed by Western blot analysis using anti-cFLIP; anti-β-actin was used as loading control. (C) Cells expressing the various Rac1 mutants were treated with anti-Fas (0.25 μg/ml) for 18 hrs in GW medium and cell survival was determined by the β-gal survival assay as described in Materials and Methods. (D–E) Cells expressing the various Rac1 mutants were treated with anti-Fas (0.25 μg/ml) for 4 hrs in GW medium and activities of capase-8 and 3 were determined in lysates using fluorometric assays as described in Materials and Methods. Results are shown as fold increase in enzyme activity over the untreated control cells. (F) Cells expressing the various Rac1 mutants were treated with anti-Fas (0.25 μg/ml) for 18 hrs in normal growth medium and cell survival was determined by the β-gal survival assay as described in Materials and Methods. (G–H) Cells were treated with anti-Fas (0.25 μg/ml) for 4 hrs in normal growth medium and activities of capase-8 and 3 were determined in lysates using fluorometric assays. Results are shown as fold increase in enzyme activity over the untreated control cells. Where applicable, data shown are Means ± S.D. of three independent experiments and P values (** <0.01; *** <0.005) were calculated by Ordinary one-way ANOVA using GraphPad Prism.